高中 | 电离平衡常数 题目答案及解析
稿件来源:高途
高中 | 电离平衡常数题目答案及解析如下,仅供参考!
选修四
第三章 水溶液中的离子平衡
第一节 弱电解质的电离
电离平衡常数
铜$\rm (I)$、乙腈$\rm ($简写为$\rm L)$的某水溶液体系中含铜物种的分布曲线如图。纵坐标$\rm (\delta)$为含铜物种占总铜的物质的量分数,总铜浓度为$1.0\times {{10}^{-3}}\text{ mol}\cdot {{\text{L}}^{-1}}$。下列描述正确的是$(\quad\ \ \ \ )$
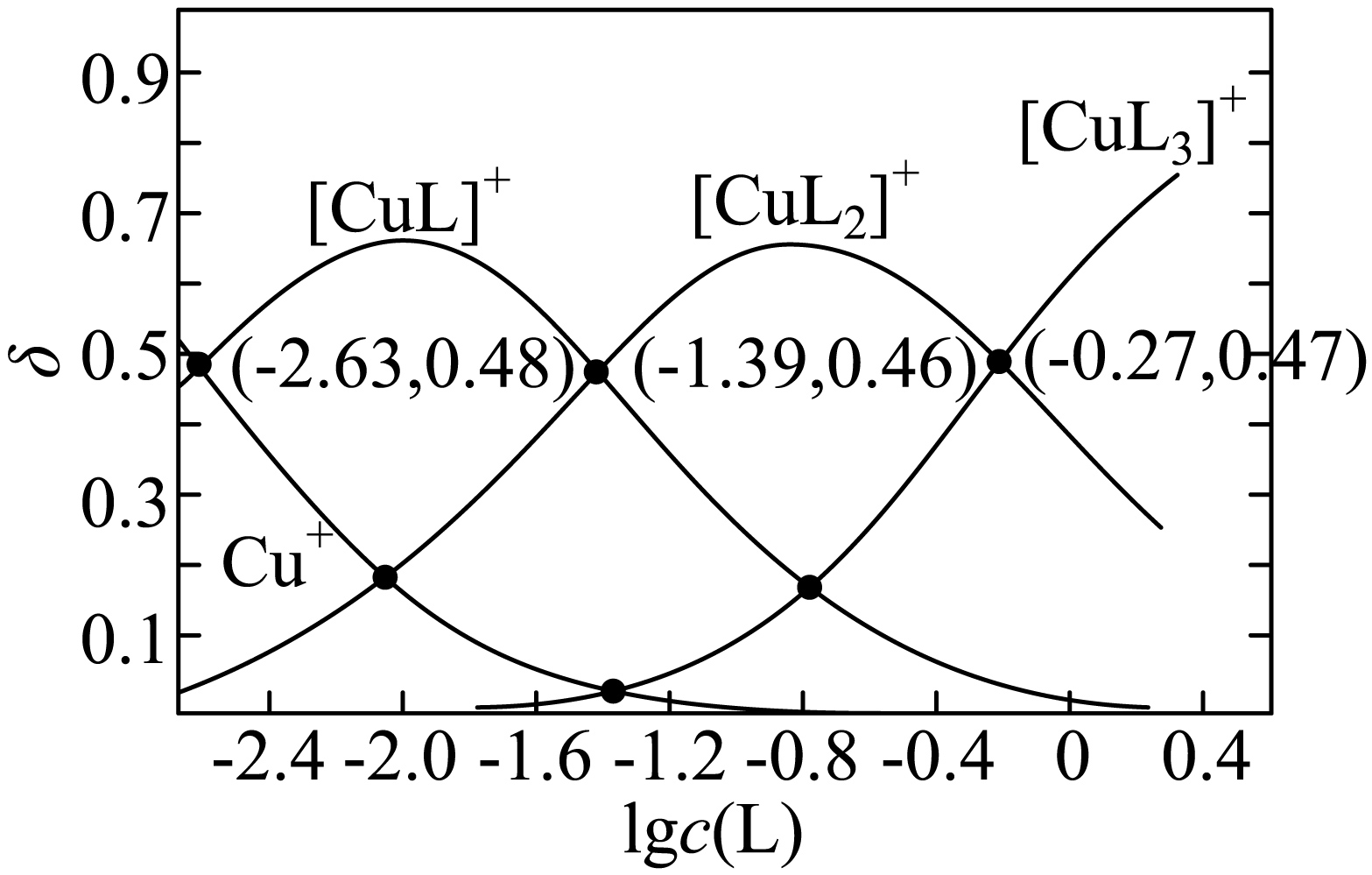
$\\text{C}{{\\text{u}}^{+}}+3\\text{L}\\rightleftharpoons {{\\left[ \\text{Cu}{{\\text{L}}_{3}} \\right]}^{+}}$的$\\lg K=0.27$
","当$c\\left( \\text{C}{{\\text{u}}^{+}} \\right)=c\\left\\{ {{[\\text{CuL}]}^{+}} \\right\\}$时,$c\\left\\{ {{\\left[ \\text{Cu}{{\\text{L}}_{2}} \\right]}^{+}} \\right\\}=2.0\\times {{10}^{-4}}\\text{ mol}\\cdot {{\\text{L}}^{-1}}$
","$n$从$\\rm 0$增加到$\\rm 2$,${{\\left[ \\text{Cu}{{\\text{L}}_{n}} \\right]}^{+}}$结合$\\rm L$的能力随之减小
","若$c\\left\\{ {{[\\text{CuL}]}^{+}} \\right\\}=c\\left\\{ {{\\left[ \\text{Cu}{{\\text{L}}_{3}} \\right]}^{+}} \\right\\}$,则$2c\\left\\{ {{\\left[ \\text{Cu}{{\\text{L}}_{2}} \\right]}^{+}} \\right\\}\\lt c\\left\\{ {{[\\text{CuL}]}^{+}} \\right\\}+3c\\left\\{ {{\\left[ \\text{Cu}{{\\text{L}}_{3}} \\right]}^{+}} \\right\\}$
"]$\rm A$.$\text{C}{{\text{u}}^{+}}+3\text{L}\rightleftharpoons {{\left[ \text{Cu}{{\text{L}}_{3}} \right]}^{+}}$的$K=\dfrac{c\left\{ {{\left[ \text{Cu}{{\text{L}}_{3}} \right]}^{+}} \right\}}{c\left( \text{C}{{\text{u}}^{+}} \right)\times {{c}^{3}}\left( \text{L} \right)}$,当图中$\delta \left\{ {{\left[ \text{Cu}{{\text{L}}_{3}} \right]}^{+}} \right\}=\delta \left( \text{C}{{\text{u}}^{+}} \right)$时,$K=\dfrac{1}{{{c}^{3}}\left( \text{L} \right)}$,$\text{lg}K=-3\text{lg}c\left( \text{L} \right)$,由图像可知,此时$-1.6\lt \text{lg}c\left( \text{L} \right)\lt -1.2$,则$\text{lg}K\rm ≠0.27$,$\rm A$错误;
$\rm B$.当$c\left( \text{C}{{\text{u}}^{+}} \right)=c\left\{ {{[\text{CuL}]}^{+}} \right\}$时,由图像可知,$\delta \left( \text{C}{{\text{u}}^{+}} \right)=\delta \left\{ {{\left[ \text{CuL} \right]}^{+}} \right\}=0.48$,$\delta \left\{ {{\left[ \text{Cu}{{\text{L}}_{3}} \right]}^{+}} \right\}$可忽略不计,则,$\delta \left\{ {{\left[ \text{Cu}{{\text{L}}_{2}} \right]}^{+}} \right\}=0.04$,$c\left\{ {{\left[ \text{Cu}{{\text{L}}_{2}} \right]}^{+}} \right\}=0.04\times 1.0\times {{10}^{-3}}=4\times {{10}^{-5}}\text{ mol/L}$,$\rm B$错误;
$\rm C$.${{\left[ \text{Cu}{{\text{L}}_{n}} \right]}^{+}}$结合$\rm L$的离子方程式为${{\left[ \text{Cu}{{\text{L}}_{n}} \right]}^{+}}+\text{L}\rightleftharpoons {{\left[ \text{Cu}{{\text{L}}_{n+1}} \right]}^{+}}$,当$\delta \left\{ {{\left[ \text{Cu}{{\text{L}}_{n}} \right]}^{+}} \right\}=\delta \left\{ {{\left[ \text{Cu}{{\text{L}}_{n+1}} \right]}^{+}} \right\}$时,$K=\dfrac{1}{c\left( \text{L} \right)}$,由图像交点可知,随着$n$变大,$c\left( \text{L} \right)$逐渐变大,则$K$值变小,说明${{\left[ \text{Cu}{{\text{L}}_{n}} \right]}^{+}}$结合$\rm L$的能力随之减小,$\rm C$正确;
$\rm D$.若$c\left\{ {{[\text{CuL}]}^{+}} \right\}=c\left\{ {{\left[ \text{Cu}{{\text{L}}_{3}} \right]}^{+}} \right\}$,由图像交点可知,$\delta \left\{ {{\left[ \text{CuL} \right]}^{+}} \right\}=\delta \left\{ {{\left[ \text{Cu}{{\text{L}}_{3}} \right]}^{+}} \right\}\lt 0.2$,$\delta \left\{ {{\left[ \text{Cu}{{\text{L}}_{2}} \right]}^{+}} \right\}\gt 0.6$,则$c\left\{ {{\left[ \text{Cu}{{\text{L}}_{2}} \right]}^{+}} \right\}\gt 2c\left\{ {{\left[ \text{Cu}{{\text{L}}_{3}} \right]}^{+}} \right\}$,故$2c\left\{ {{\left[ \text{Cu}{{\text{L}}_{2}} \right]}^{+}} \right\}\gt c\left\{ {{\left[ \text{CuL} \right]}^{+}} \right\}+3c\left\{ {{\left[ \text{Cu}{{\text{L}}_{3}} \right]}^{+}} \right\}$,$\rm D$错误;
故选:$\rm C$
高中 | 电离平衡常数题目答案及解析(完整版)
