高中 | 盐溶液微粒间的三大守恒原理的理解及应用 题目答案及解析
稿件来源:高途
高中 | 盐溶液微粒间的三大守恒原理的理解及应用题目答案及解析如下,仅供参考!
选修四
第三章 水溶液中的离子平衡
第三节 盐类的水解
盐溶液微粒间的三大守恒原理的理解及应用
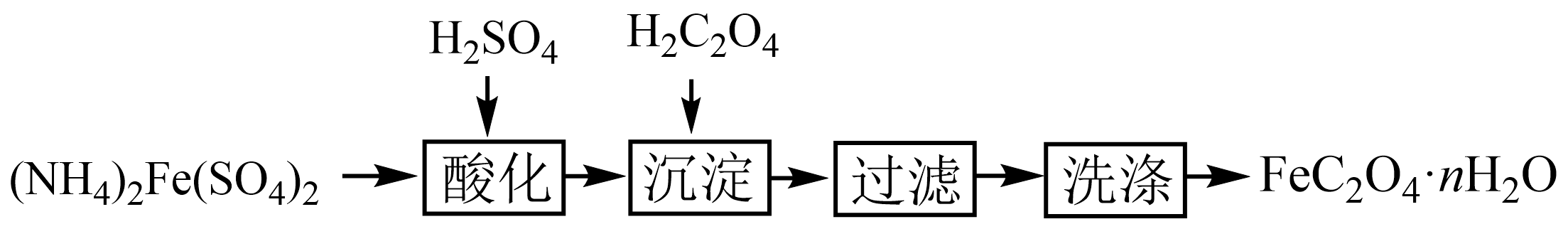
实验室通过下列过程制取草酸亚铁晶体:
已知:$K_{\mathrm{a1}}\left( \mathrm{NH}_{3}\cdot\mathrm{H}_{2}\mathrm{O}\right)=1.8\times10^{-5}$、$K_{\mathrm{a2}}\left( \mathrm{NH}_{3}\cdot\mathrm{H}_{2}\mathrm{O}\right)=1.8\times10^{-5}$。下列说法不正确的是$(\qquad)$
$\\text{pH}=4$的${{\\text{H}}_{2}}{{\\text{C}}_{2}}{{\\text{O}}_{4}}$溶液中:$\\textit{c}(\\text{C}_{2}\\text{O}_{4}^{2-})\\gt\\textit{c}(\\text{H}\\text{C}_{2}\\text{O}_{4}^{-})$
","“酸化”后的溶液中:$\\textit{c}(\\text{NH}_{4}^{+})+2\\textit{c}(\\text{F}\\text{e}^{2+})\\lt2\\textit{c}(\\text{SO}_{4}^{2-})$
","“沉淀”后的上层清液中:$\\textit{c}(\\text{NH}_{4}^{+})+\\textit{c}(\\text{N}\\text{H}_{3}\\cdot\\text{H}_{2}\\text{O})=\\textit{c}(\\text{SO}_{4}^{2-})$
","水洗后,再用乙醇洗涤有利于晶体快速干燥
"]$\rm (NH_{4})_{2}Fe(SO_{4})_{2}$加入硫酸酸化得到$\rm Fe^{2+}$,加入草酸生成$\rm FeC_{2}O_{4}$沉淀,过滤、洗涤得到草酸亚铁晶体,据此回答。
$\rm A$.$\text{pH}=4$的${{\text{H}}_{2}}{{\text{C}}_{2}}{{\text{O}}_{4}}$溶液中$\textit{c}\left( \text{H}^{+}\right)=10^{-4}\ \text{mol/L}$,$\textit{K}_{\text{a}2}(\text{H}_{2}\text{C}_{2}\text{O}_{4})=\dfrac{\textit{c}(\text{C}_{2}\text{O}_{4}^{2-})\textit{c}(\text{H}^{+})}{\textit{c}(\text{H}\text{C}_{2}\text{O}_{4}^{-})}=1.5\times10^{-4}$,$\dfrac{\textit{c}(\text{C}_{2}\text{O}_{4}^{2-})}{\textit{c}(\text{H}\text{C}_{2}\text{O}_{4}^{-})}=\dfrac{\textit{K}_{\text{a}2}(\text{H}_{2}\text{C}_{2}\text{O}_{4})}{\textit{c}(\text{H}^{+})}=\dfrac{1.5\times10^{-4}}{10^{-4}}=1.5$,即$\textit{c}(\text{C}_{2}\text{O}_{4}^{2-})=1.5\textit{c}(\text{H}\text{C}_{2}\text{O}_{4}^{-})$,即$\textit{c}(\text{C}_{2}\text{O}_{4}^{2-})\gt\textit{c}(\text{H}\text{C}_{2}\text{O}_{4}^{-})$,$\rm A$正确;
$\rm B$.根据电荷守恒:$\textit{c}(\text{NH}_{4}^{+})+2\textit{c}(\text{F}\text{e}^{2+})+\textit{c}(\text{H}^{+})=2\textit{c}(\text{SO}_{4}^{2-})+\textit{c}(\text{O}\text{H}^{-})$,溶液显酸性,$\textit{c}\left( \text{H}^{+}\right)>\textit{c}\left( \text{O}\text{H}^{-}\right)$,则$\textit{c}(\text{NH}_{4}^{+})+2\textit{c}(\text{F}\text{e}^{2+})\lt2\textit{c}(\text{SO}_{4}^{2-})$,$\rm B$正确;
$\rm C$.“沉淀”后的上层清液中溶质为$\rm (NH_{4})_{2}SO_{4}$和过量的${{\text{H}}_{2}}{{\text{C}}_{2}}{{\text{O}}_{4}}$,由物料守恒得:$\textit{c}(\text{NH}_{4}^{+})+\textit{c}(\text{N}\text{H}_{3}\cdot\text{H}_{2}\text{O})=2\textit{c}(\text{SO}_{4}^{2-})$,$\rm C$错误;
$\rm D$.乙醇与水互溶,且乙醇易挥发,用乙醇洗可带走水分,有利于晶体快速干燥,$\rm D$正确;
故选:$\rm C$
高中 | 盐溶液微粒间的三大守恒原理的理解及应用题目答案及解析(完整版)
