高中 | 盐溶液微粒间的三大守恒原理的理解及应用 题目答案及解析
稿件来源:高途
高中 | 盐溶液微粒间的三大守恒原理的理解及应用题目答案及解析如下,仅供参考!
选修四
第三章 水溶液中的离子平衡
第三节 盐类的水解
盐溶液微粒间的三大守恒原理的理解及应用
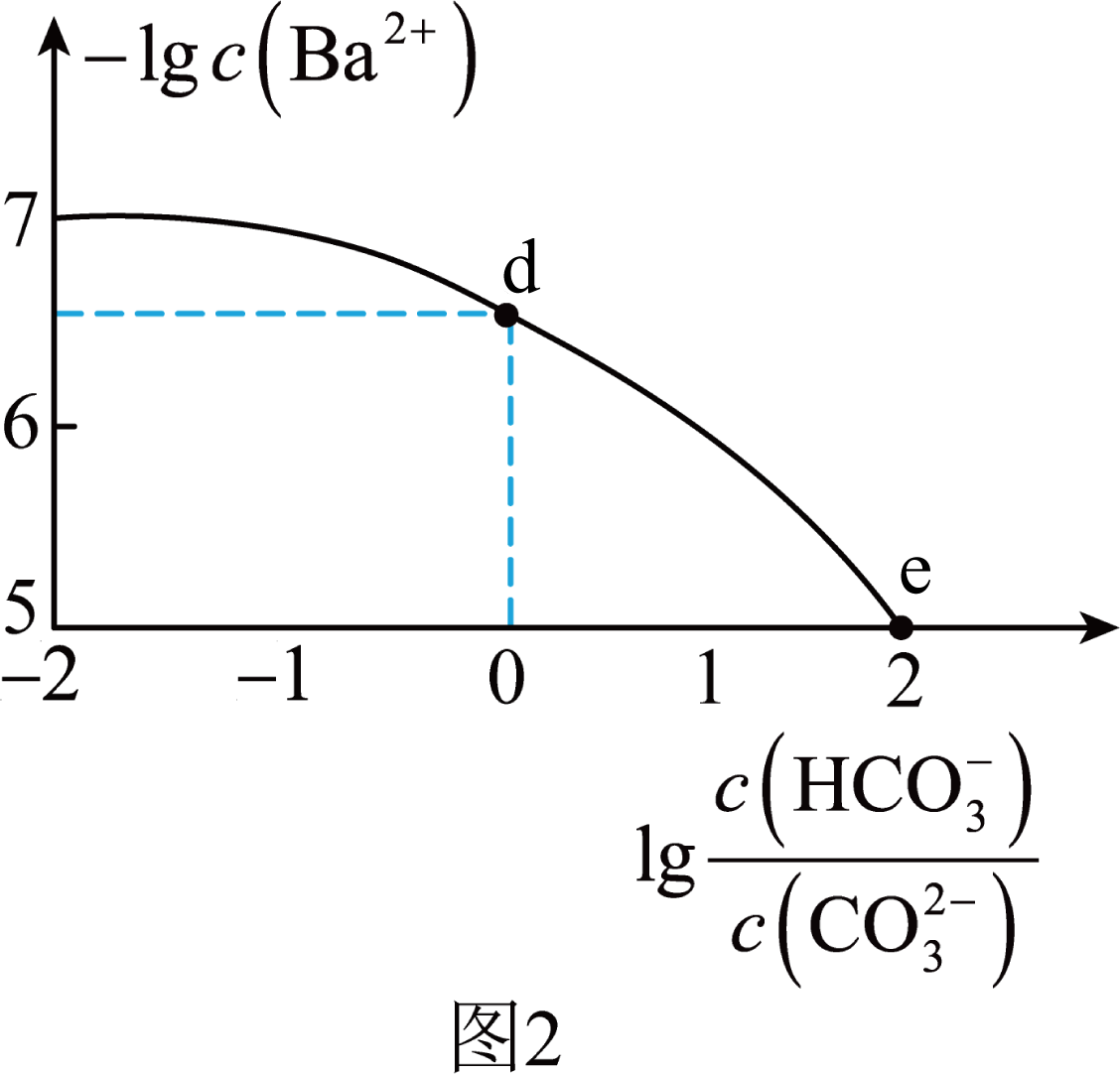
室温下,${{\text{H}}_{2}}\text{C}{{\text{O}}_{3}}$溶液中各含碳微粒的物质的量分数与$\rm pH$的关系如图$\rm 1$所示;向$\text{N}{{\text{a}}_{2}}\text{C}{{\text{O}}_{3}}$、$\text{NaHC}{{\text{O}}_{3}}$的混合溶液中滴加$\text{BaC}{{\text{l}}_{2}}$溶液,所得溶液中$-\lg c \left( \text{B}{{\text{a}}^{2+}} \right)$与$\lg \dfrac{c\left( \text{HCO}_{3}^{-} \right)}{c\left( \text{CO}_{3}^{2-} \right)}$的关系如图$\rm 2$所示。已知:标准状况下,$\rm 1$体积$\text{C}{{\text{O}}_{2}}$可溶于约$\rm 1$体积水中,${{\text{H}}_{2}}\text{O}+\text{C}{{\text{O}}_{2}}\rightleftharpoons {{\text{H}}_{2}}\text{C}{{\text{O}}_{3}}\qquad K=\dfrac{1}{600}$。下列说法错误的是$(\qquad)$
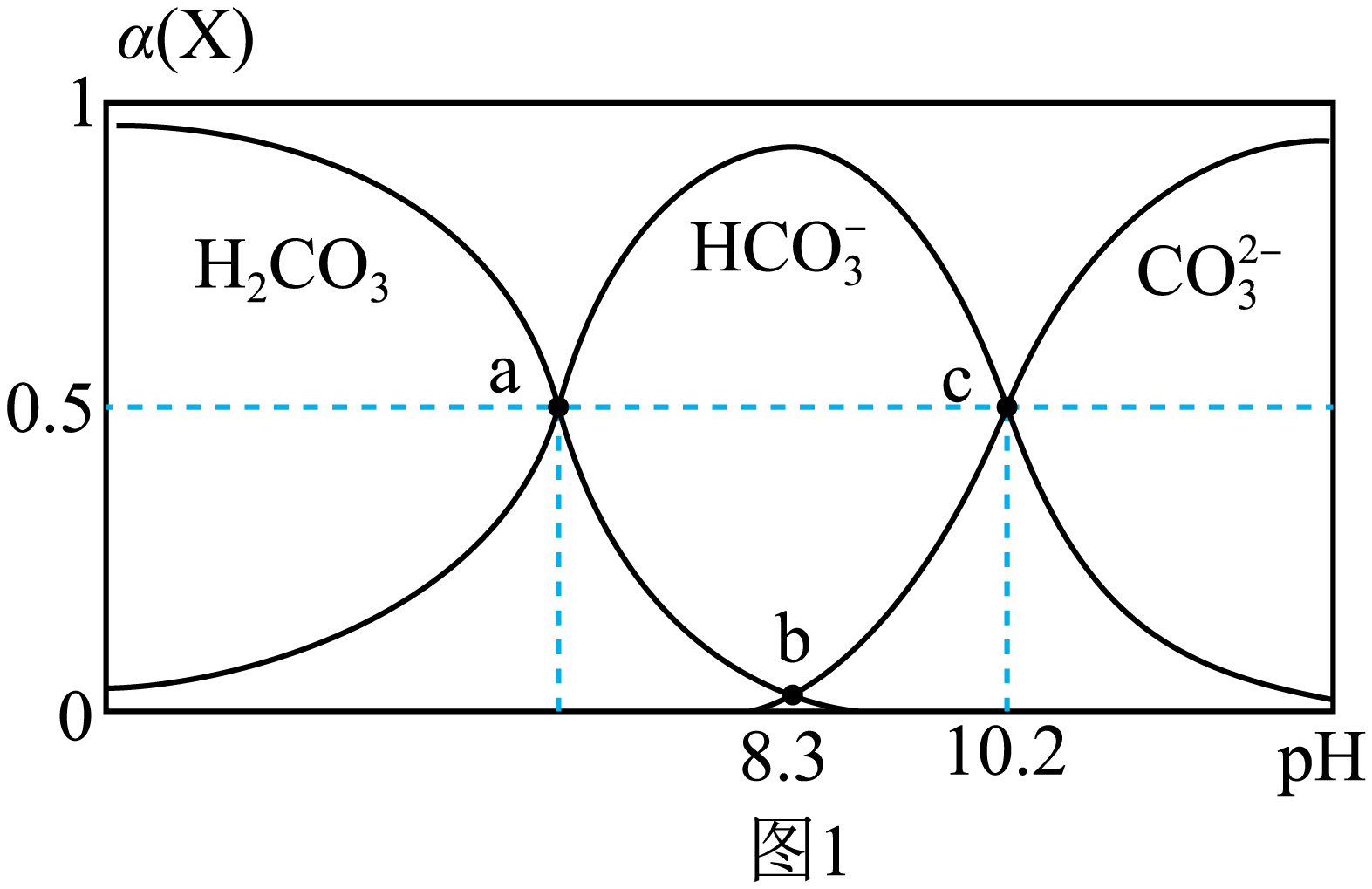
${{K}_{\\text{a1}}}\\left( {{\\text{H}}_{2}}\\text{C}{{\\text{O}}_{3}} \\right)$的数量级为${{10}^{-7}}$
","$\\text{d}$点溶液中存在:$c\\left( \\text{N}{{\\text{a}}^{+}} \\right)+2c\\left( \\text{B}{{\\text{a}}^{2+}} \\right)\\gt 3c\\left( \\text{HCO}_{3}^{-} \\right)+c\\left( \\text{C}{{\\text{l}}^{-}} \\right)$
","$\\text{d}\\to \\text{e}$点过程中,$\\dfrac{c\\left( \\text{CO}_{3}^{2-} \\right)\\cdot c\\left( {{\\text{H}}^{+}} \\right)}{c\\left( {{\\text{H}}_{2}}\\text{C}{{\\text{O}}_{3}} \\right)}$一直增大
","饱和${{\\text{H}}_{2}}\\text{C}{{\\text{O}}_{3}}$溶液中$c\\left( {{\\text{H}}_{2}}\\text{C}{{\\text{O}}_{3}} \\right)$约为$7.4\\times {{10}^{-5}}\\;\\rm \\text{mol}\\cdot {{\\text{L}}^{-1}}$
"]根据图示,$c\left( \text{CO}_{3}^{2-} \right)=c\left( \text{HCO}_{3}^{-} \right)$时,$\rm pH=10.2$,即${{K}_{\text{a2}}}\left( {{\text{H}}_{2}}\text{C}{{\text{O}}_{3}} \right)=1\times {{10}^{-10.2}}$;$c\left( \text{CO}_{3}^{2-} \right)=c\left( {{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}} \right)$时,$\rm pH=8.3$,${{K}_{\text{a1}}}\left( {{\text{H}}_{2}}\text{C}{{\text{O}}_{3}} \right)\times {{K}_{\text{a2}}}\left( {{\text{H}}_{2}}\text{C}{{\text{O}}_{3}} \right) =\dfrac{c\left( \text{CO}_{3}^{2-} \right){{c}^{2}}\left( {{\text{H}}^{+}} \right)}{c\left( {{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}} \right)}=1\times {{10}^{-16.6}}$。
$\rm A$.根据图$\rm 1$,${{K}_{\text{a2}}}\left( {{\text{H}}_{2}}\text{C}{{\text{O}}_{3}} \right)=1\times {{10}^{-10.2}}$,${{K}_{\text{a1}}}\left( {{\text{H}}_{2}}\text{C}{{\text{O}}_{3}} \right)\times {{K}_{\text{a2}}}\left( {{\text{H}}_{2}}\text{C}{{\text{O}}_{3}} \right)={{10}^{-16.6}}$,${{K}_{\text{a1}}}\left( {{\text{H}}_{2}}\text{C}{{\text{O}}_{3}} \right)\rm ={{10}^{-6.4}}$ ,所以${{K}_{\text{a1}}}\left( {{\text{H}}_{2}}\text{C}{{\text{O}}_{3}} \right)$数量级为${{10}^{-7}}$,故$\rm A$正确;
$\rm B$.根据电荷守恒,$\text{d}$点溶液中$c\left( \text{N}{{\text{a}}^{+}} \right)+2c\left( \text{B}{{\text{a}}^{2+}} \right)+c\left( {{\text{H}}^{+}} \right)=2c\left( \text{CO}_{3}^{2-} \right)+c\left( \text{HCO}_{3}^{-} \right)+c\left( \text{C}{{\text{l}}^{-}} \right)+c\left( \text{O}{{\text{H}}^{-}} \right)$,根据图$\rm 2$,$\text{d}$点溶液$c\left( \text{CO}_{3}^{2-} \right)=c\left( \text{HCO}_{3}^{-} \right)$,溶液中含有碳酸根离子、碳酸氢根离子,溶液呈碱性,${c\rm (H^{+})}\lt c\rm (OH^{-})$,所以$c\left( \text{N}{{\text{a}}^{+}} \right)+2c\left( \text{B}{{\text{a}}^{2+}} \right)\gt 3c\left( \text{HCO}_{3}^{-} \right)+c\left( \text{C}{{\text{l}}^{-}} \right)$,故$\rm B$正确;
$\rm C$.$\text{d}\to \text{e}$点过程中$\dfrac{c\left( \text{HCO}_{3}^{-} \right)}{c\left( \text{CO}_{3}^{2-} \right)}$增大,$\dfrac{1}{K{{}_{\rm a2}}}=\dfrac{c\left( \text{HCO}_{3}^{-} \right)}{c\left( \text{CO}_{3}^{2-} \right)c\left( {{\text{H}}^{+}} \right)}$不变,所以$ c\rm(H^{+})$增大,${{K}_{\text{a1}}}\left( {{\text{H}}_{2}}\text{C}{{\text{O}}_{3}} \right)\times {{K}_{\text{a2}}}\left( {{\text{H}}_{2}}\text{C}{{\text{O}}_{3}} \right) =\dfrac{c\left( \text{CO}_{3}^{2-} \right){{c}^{2}}\left( {{\text{H}}^{+}} \right)}{c\left( {{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}} \right)}$不变,则$\dfrac{c\left( \text{CO}_{3}^{2-} \right)\cdot c\left( {{\text{H}}^{+}} \right)}{c\left( {{\text{H}}_{2}}\text{C}{{\text{O}}_{3}} \right)}$减小,故$\rm C$错误;
$\rm D$.${{\text{H}}_{2}}\text{O}+\text{C}{{\text{O}}_{2}}\rightleftharpoons {{\text{H}}_{2}}\text{C}{{\text{O}}_{3}}\qquad K=\dfrac{1}{600}$,即$\dfrac{c\left( {{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}} \right)}{c\left( \text{C}{{\text{O}}_{\text{2}}} \right)}=\dfrac{1}{600}$,标准状况下,$\rm 1$体积$\text{C}{{\text{O}}_{2}}$可溶于约$\rm 1$体积水中,饱和${{\text{H}}_{2}}\text{C}{{\text{O}}_{3}}$溶液中$c\rm (CO_{2})=\dfrac{1}{22.4}\text{ mol/L}$,所以$c\left( {{\text{H}}_{2}}\text{C}{{\text{O}}_{3}} \right)\rm =7.4\times {{10}^{-5}}\;\rm \text{mol}\cdot {{\text{L}}^{-1}}$,故$\rm D$正确。
故选:$\rm C$
高中 | 盐溶液微粒间的三大守恒原理的理解及应用题目答案及解析(完整版)
