| 化学平衡常数 题目答案及解析
稿件来源:高途
| 化学平衡常数题目答案及解析如下,仅供参考!
选修四
第二章 化学反应速率和化学平衡
第三节 化学平衡
化学平衡常数
常温下,溶液中$\text{A}{{\text{l}}^{\text{3+}}}、\text{Z}{{\text{n}}^{\text{2+}}}、\text{C}{{\text{d}}^{\text{2+}}}$以氢氧化物形式沉淀时,$\text{-lg}\left[ c\text{(X)/}\left( \text{mol}\cdot {{\text{L}}^{\text{-1}}} \right) \right]$与$\text{-lg}\left[ c\left( {{\text{H}}^{+}} \right)\text{/}\left( \text{mol}\cdot {{\text{L}}^{\text{-1}}} \right) \right]$的关系如图$\rm [$其中$\rm X$代表$\text{A}{{\text{l}}^{\text{3+}}}、\text{Z}{{\text{n}}^{\text{2+}}}、\text{C}{{\text{d}}^{\text{2+}}}、\text{Al(OH)}_{\text{4}}^{-}、\text{Zn(OH)}_{\text{4}}^{\text{2-}}$或$\text{Cd(OH)}_{4}^{\text{2-}}\rm ]$。已知:${{K}_{\text{sp}}}\left[ \text{Zn(OH}{{\text{)}}_{\text{2}}} \right]\lt {{K}_{\text{sp}}}\left[ \text{Cd(OH}{{\text{)}}_{\text{2}}} \right]$,$\text{Zn(OH}{{\text{)}}_{\text{2}}}$比$\text{Cd(OH}{{\text{)}}_{\text{2}}}$更易与碱反应,形成$\text{M(OH)}_{\text{4}}^{\text{2-}}$;溶液中$c\text{(X)}\le \text{1}{{\text{0}}^{\text{-5}}}\text{ mol}\cdot {{\text{L}}^{\text{-1}}}$时,$\rm X$可忽略不计。
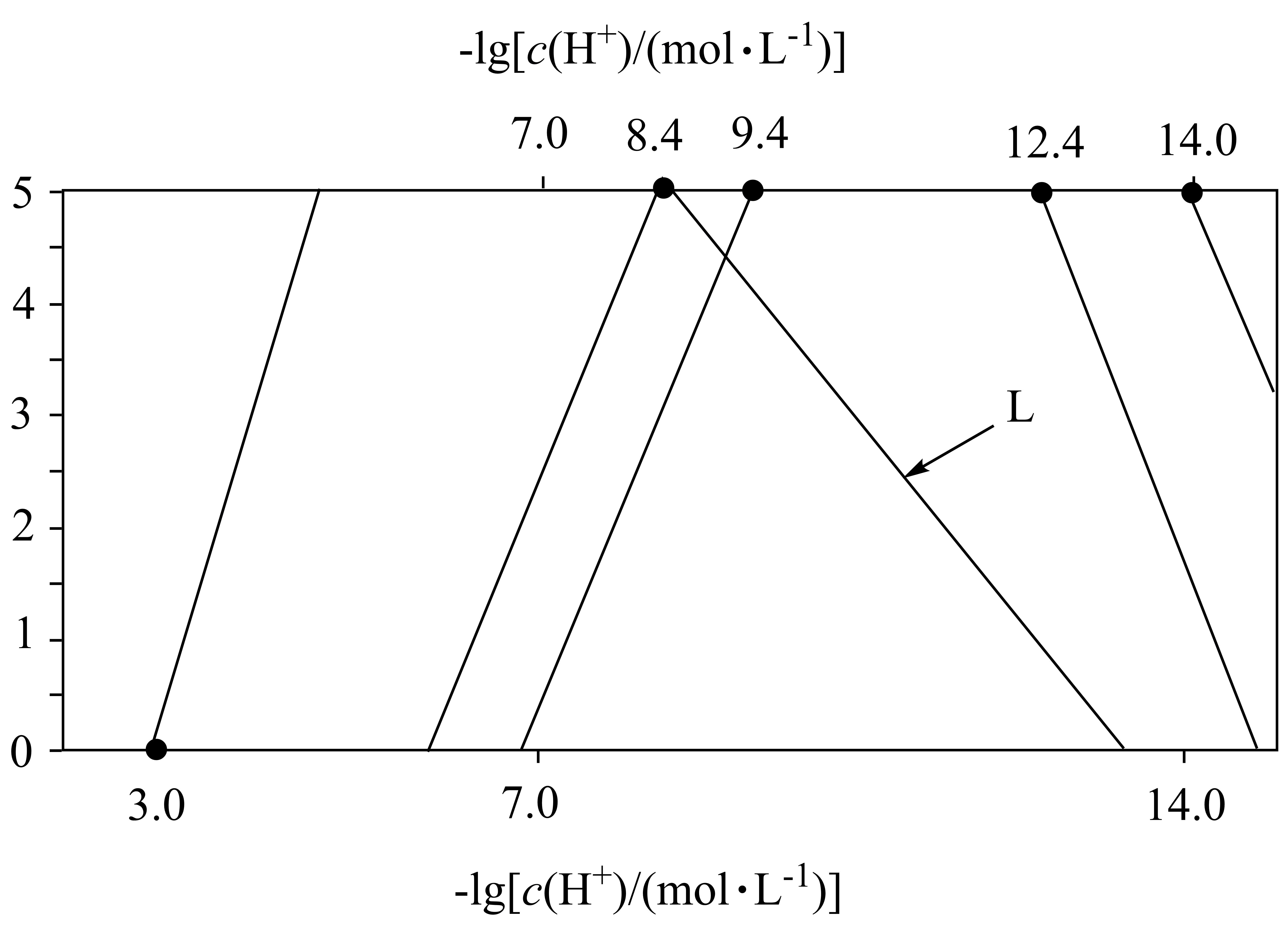
下列说法错误的是$(\quad\ \ \ \ )$
$\\rm L$为$-\\lg c\\left[ \\text{Al(OH)}_{\\text{4}}^{-} \\right]$与$-\\lg c\\left( {{\\text{H}}^{+}} \\right)$的关系曲线
","$\\text{Z}{{\\text{n}}^{\\text{2+}}}\\text{+4O}{{\\text{H}}^{-}}\\text{=Zn(OH)}_{\\text{4}}^{\\text{2-}}$的平衡常数为$\\text{1}{{\\text{0}}^{\\text{11}\\text{.2}}}$
","调节$\\text{NaOH}$溶液浓度,通过碱浸可完全分离$\\text{Cd(OH}{{\\text{)}}_{\\text{2}}}$和$\\text{Al(OH}{{\\text{)}}_{\\text{3}}}$
","调节溶液$\\text{pH}$为$\\text{4}\\text{.7 }\\sim \\text{ 6}\\text{.4}$,可将浓度均为$\\text{0}{.1\\;\\rm mol}\\cdot {{\\text{L}}^{\\text{-1}}}$的$\\text{Z}{{\\text{n}}^{\\text{2+}}}$和$\\text{A}{{\\text{l}}^{\\text{3+}}}$完全分离
"]$\text{Z}{{\text{n}}^{2+}}$和$\text{C}{{\text{d}}^{\text{2+}}}$沉淀形成$\text{Zn}{{\left( \text{OH} \right)}_{\text{2}}}$和$\text{Cd}{{\left( \text{OH} \right)}_{\text{2}}}$,$\text{A}{{\text{l}}^{\text{3+}}}$沉淀形成$\text{Al}{{\left( \text{OH} \right)}_{\text{3}}}$,则$\text{Z}{{\text{n}}^{2+}}$和$\text{C}{{\text{d}}^{\text{2+}}}$的曲线平行,根据${{K}_{\text{sp}}}\left[ \text{Zn(OH}{{\text{)}}_{\text{2}}} \right]\lt {{K}_{\text{sp}}}\left[ \text{Cd(OH}{{\text{)}}_{\text{2}}} \right]$,$\text{Zn(OH}{{\text{)}}_{\text{2}}}$比$\text{Cd(OH}{{\text{)}}_{\text{2}}}$更易与碱反应,因此$\text{Zn(OH)}_{\text{4}}^{\text{2-}}$生成的$\rm pH$低于$\text{Cd(OH)}_{4}^{\text{2-}}$,故从左到右曲线依次为:$\text{A}{{\text{l}}^{\text{3+}}}、\text{Z}{{\text{n}}^{\text{2+}}}、\text{C}{{\text{d}}^{\text{2+}}}、\text{Al(OH)}_{\text{4}}^{-}、\text{Zn(OH)}_{\text{4}}^{\text{2-}}$或$\text{Cd(OH)}_{4}^{\text{2-}}$。如图可知:$\text{A}{{\text{l}}^{\text{3+}}}\text{+3O}{{\text{H}}^{-}}\text{=Al(OH)}_{3}^{{}}$的平衡常数为${{K}_{\text{sp}}}[\text{Al}\left(\text{OH}\right)_{3}^{{}}]=c[\!\!\text{ A}{{\text{l}}^{\text{3+}}}]\cdot {{c}^{3}}[\!\!\text{ O}{{\text{H}}^{-}}]\!\!\text{ =1}{{\text{0}}^{-0}}\times {{[\!\!\text{ 1}{{\text{0}}^{-\left( 14-3 \right)}}]}^{3}}\text{=1}{{\text{0}}^{-0-3\times 11}}\text{=1}{{\text{0}}^{-33}}$,$\text{Z}{{\text{n}}^{\text{2+}}}\text{+2O}{{\text{H}}^{-}}\text{=Zn(OH)}_{2}^{{}}$的平衡常数为${{K}_{\text{sp}}}[\!\!\text{ Zn}\left( \text{OH} \right)_{\text{2}}^{{}}]\!\!\text{ =}c[\!\!\text{ Z}{{\text{n}}^{\text{2+}}}]\cdot {{c}^{\text{2}}}[\!\!\text{ O}{{\text{H}}^{-}}]\!\!\text{ =1}{{\text{0}}^{-5}}\times {{[\!\!\text{ 1}{{\text{0}}^{-\left( 14-8.4 \right)}}]}^{2}}\text{=1}{{\text{0}}^{-5-2\times 5.6}}\text{=1}{{\text{0}}^{-16.2}}$,$\text{C}{{\text{d}}^{\text{2+}}}\text{+2O}{{\text{H}}^{-}}\text{=Cd(OH)}_{2}^{{}}$的平衡常数为${{K}_{\text{sp}}}[\!\!\text{ Cd}\left( \text{OH} \right)_{\text{2}}^{{}}]\!\!\text{ =}c[\!\!\text{ C}{{\text{d}}^{\text{2+}}}]\cdot {{c}^{\text{2}}}[\!\!\text{ O}{{\text{H}}^{-}}]\!\!\text{ =1}{{\text{0}}^{-5}}\times {{[\!\!\text{ 1}{{\text{0}}^{-\left( 14-9.4 \right)}}]}^{2}}\text{=1}{{\text{0}}^{-5-2\times 4.6}}\text{=1}{{\text{0}}^{-14.2}}$,$\text{Al}\left( \text{OH} \right)_{3}^{{}}\text{+O}{{\text{H}}^{-}}\text{=Al(OH)}_{4}^{-}$的平衡常数为$K=\dfrac{{{10}^{-5}}}{{{10}^{-\left( 14-8.4 \right)}}}\text{=1}{{\text{0}}^{-5+5.6}}\text{=1}{{\text{0}}^{0.6}}$,$\text{Zn}\left( \text{OH} \right)_{2}^{{}}\text{+2O}{{\text{H}}^{-}}\text{=Zn(OH)}_{4}^{2-}$的平衡常数为$K=\dfrac{{{10}^{-5}}}{{{10}^{-\left( 14-12.4 \right)}}}\text{=1}{{\text{0}}^{-5+1.6}}\text{=1}{{\text{0}}^{-3.4}}$,$\text{Cd}\left( \text{OH} \right)_{2}^{{}}\text{+2O}{{\text{H}}^{-}}\text{=Cd(OH)}_{4}^{2-}$的平衡常数为$K=\dfrac{{{10}^{-5}}}{{{10}^{-\left( 14-14 \right)}}}\text{=1}{{\text{0}}^{-5+0}}\text{=1}{{\text{0}}^{-5}}$。据此分析:
$\rm A$.据分析,$\rm L$为$-\lg c\left[ \text{Al(OH)}_{\text{4}}^{-} \right]$与$-\lg c\left( {{\text{H}}^{+}} \right)$的关系曲线,故$\rm A$正确;
$\rm B$.如图可知,$\text{Z}{{\text{n}}^{\text{2+}}}\text{+2O}{{\text{H}}^{-}}\text{=Zn(OH)}_{2}^{{}}$的平衡常数为${{K}_{\text{sp}}}[\!\!\text{ Zn}\left( \text{OH} \right)_{\text{2}}^{{}}]\!\!\text{ =}c[\!\!\text{ Z}{{\text{n}}^{\text{2+}}}]\cdot {{c}^{\text{2}}}[\!\!\text{ O}{{\text{H}}^{-}}]\!\!\text{ =1}{{\text{0}}^{-5}}\times {{[\!\!\text{ 1}{{\text{0}}^{-\left( 14-8.4 \right)}}]}^{2}}\text{=1}{{\text{0}}^{-5-2\times 5.6}}\text{=1}{{\text{0}}^{-16.2}}$,则$\text{Z}{{\text{n}}^{\text{2+}}}\text{+4O}{{\text{H}}^{-}}\text{=Zn(OH)}_{\text{4}}^{\text{2-}}$的平衡常数为$K=\dfrac{c\left[ \text{Zn}\left( \text{OH} \right)_{\text{4}}^{\text{2-}} \right]}{c\left[ \text{Z}{{\text{n}}^{\text{2+}}}]\cdot {{c}^{\text{4}}}[\!\!\text{ O}{{\text{H}}^{-}} \right]}=\dfrac{c\left[ \text{Zn}\left( \text{OH} \right)_{\text{4}}^{\text{2-}} \right]}{{{K}_{\text{sp}}}[\!\!\text{ Zn}\left( \text{OH} \right)_{2}^{{}}]\cdot {{c}^{2}}[\!\!\text{ O}{{\text{H}}^{-}}]}=\dfrac{{{10}^{-5}}}{{{10}^{-16.2}}\times {{\left[ {{10}^{-\left( 14-12.4 \right)}} \right]}^{2}}}\text{=1}{{\text{0}}^{-5+16.2+2\times 1.6}}\text{=1}{{\text{0}}^{14.4}}$,故$\rm B$错误;
$\rm C$.如图可知,$\rm pH=14$时$\text{Cd}\left( \text{OH} \right)_{2}^{{}}$开始溶解,$\rm pH=8.4$时$\text{Al}\left( \text{OH} \right)_{\text{3}}^{{}}$开始溶解,且$\rm pH=14$时$c[\text{Al(OH)}_{\text{4}}^{-}]\gt \text{1 mol/L}$,即可认为$\text{Al}\left( \text{OH} \right)_{\text{3}}^{{}}$完全溶解,并转化为$\text{Al(OH)}_{\text{4}}^{-}$,因此调节$\text{NaOH}$溶液浓度,通过碱浸可完全分离$\text{Cd}\left( \text{OH} \right)_{2}^{{}}$和$\text{Al(OH}{{\text{)}}_{\text{3}}}$,故$\rm C$正确;
$\rm D$.$\text{0}\text{.1 mol}\cdot {{\text{L}}^{\text{-1}}}$的$\text{Z}{{\text{n}}^{\text{2+}}}$开始沉淀$\rm pH$为$14+\lg \left( \sqrt{\dfrac{{{10}^{-16.2}}}{0.1}} \right)=6.4$,$\text{0}\text{.1 mol}\cdot {{\text{L}}^{\text{-1}}}$的$\text{A}{{\text{l}}^{\text{3+}}}$完全沉淀$\rm pH$为$14+\lg \left( \sqrt[3]{\dfrac{{{10}^{-33}}}{{{10}^{-5}}}} \right)=4.7$,因此调节溶液$\text{pH}$为$\text{4}\text{.7}\sim \text{6}\text{.4}$,可将浓度均为$\text{0}\text{.1 mol}\cdot {{\text{L}}^{\text{-1}}}$的$\text{Z}{{\text{n}}^{\text{2+}}}$和$\text{A}{{\text{l}}^{\text{3+}}}$完全分离,故$\rm D$正确;
故选:$\rm B$
| 化学平衡常数题目答案及解析(完整版)
