高中 | 盐溶液微粒间的三大守恒原理的理解及应用 题目答案及解析
稿件来源:高途
高中 | 盐溶液微粒间的三大守恒原理的理解及应用题目答案及解析如下,仅供参考!
选修四
第三章 水溶液中的离子平衡
第三节 盐类的水解
盐溶液微粒间的三大守恒原理的理解及应用
$\textit{T}\;\rm ^\circ\rm C,\text{AgBr}$悬浊液$\rm ($含足量$\text{AgBr}$固体$\rm )$加$\text{N}\text{a}_{2}\text{S}_{2}\text{O}_{3}$固体,发生反应$\text{A}{{\text{g}}^{+}}+{{\text{S}}_{\text{2}}}\text{O}_{\text{3}}^{\text{2-}}\rightleftharpoons {{\left[ \text{Ag}\left( {{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}} \right) \right]}^{-}}$和${{\left[ \text{Ag}\left( {{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}} \right) \right]}^{-}}+{{\text{S}}_{\text{2}}}\text{O}_{\text{3}}^{\text{2-}}\rightleftharpoons {{\left[ \text{Ag}{{\left( {{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}} \right)}_{\text{2}}} \right]}^{\text{3-}}}$,$\text{lgM}$、$\text{lgN}$与$\text{lg}c\left( \text{S}_{\text{2}}\text{O}_{\text{3}}^{\text{2-}}\right)$的关系如图所示,$\text{M}$代表$\textit{c}\left( \text{A}\text{g}^{+}\right)$或$\textit{c}\left( \text{B}{{\text{r}}^{-}} \right)$,$\rm N$代表$\dfrac{\textit{c}\left( \text{A}{{\text{g}}^{+}} \right)}{\textit{c}\left\{ {{\left[ \text{Ag}\left( {{\text{S}}_{2}}{{\text{O}}_{3}} \right) \right]}^{-}} \right\}}$或$\dfrac{\textit{c}\left( \text{A}{{\text{g}}^{+}} \right)}{\textit{c}\left\{ {{\left[ \text{Ag}{{\left( {{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}} \right)}_{\text{2}}} \right]}^{\text{3-}}} \right\}}$。已知${{\textit{K}}_{\text{sp}}}\left( \text{AgBr} \right)={{10}^{-12.2}}$。下列说法错误的是$(\qquad)$
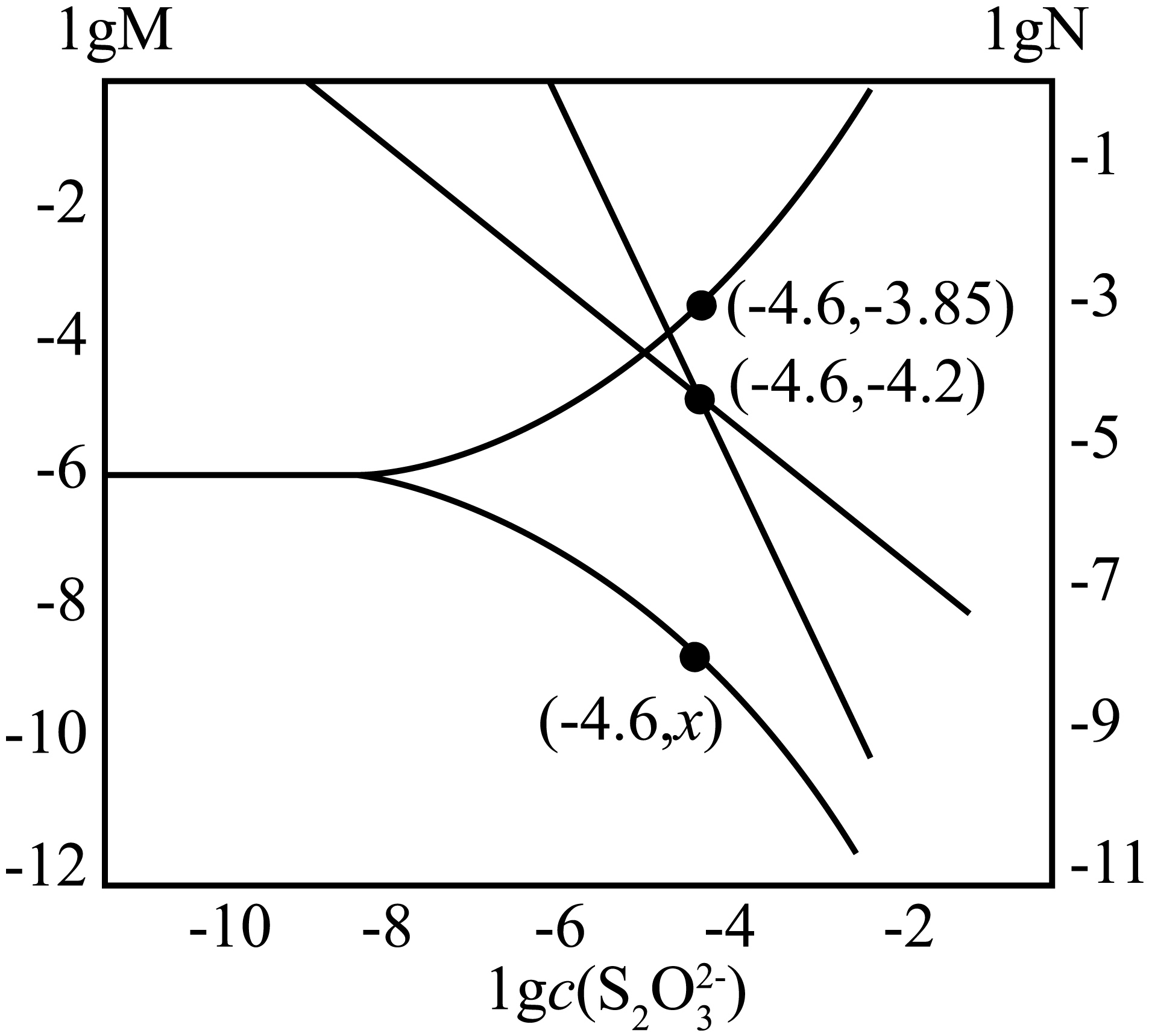
$\\textit{x}=-8.35$
","$\\text{AgBr}\\left( \\text{s} \\right)\\text{+2}\\,{{\\text{S}}_{\\text{2}}}\\text{O}_{\\text{3}}^{\\text{2-}}\\left( \\text{aq} \\right)\\rightleftharpoons {{\\left[ \\text{Ag}{{\\left( {{\\text{S}}_{\\text{2}}}{{\\text{O}}_{\\text{3}}} \\right)}_{\\text{2}}} \\right]}^{\\text{3-}}}\\left( \\text{aq} \\right)\\text{+B}{{\\text{r}}^{-}}\\left( \\text{aq} \\right)$的平衡常数$\\textit{K}={{10}^{1.2}}$
","$\\textit{c}\\left( {{\\text{S}}_{\\text{2}}}\\text{O}_{\\text{3}}^{\\text{2-}} \\right)=\\textit{c}\\left\\{ {{\\left[ \\text{Ag}{{\\left( {{\\text{S}}_{\\text{2}}}{{\\text{O}}_{\\text{3}}} \\right)}_{\\text{2}}} \\right]}^{\\text{3-}}} \\right\\}$时,$\\left( \\text{1}{{\\text{0}}^{1.2}}-1 \\right)\\textit{c}\\left( {{\\text{S}}_{\\text{2}}}\\text{O}_{\\text{3}}^{\\text{2-}} \\right)=\\textit{c}\\left( \\text{A}{{\\text{g}}^{+}} \\right)+\\textit{c}\\left\\{ {{\\left[ \\text{Ag}\\left( {{\\text{S}}_{\\text{2}}}{{\\text{O}}_{\\text{3}}} \\right) \\right]}^{-}} \\right\\}$
","$\\textit{c}\\left( {{\\text{S}}_{\\text{2}}}\\text{O}_{\\text{3}}^{\\text{2-}} \\right)=\\textit{c}\\left\\{ {{\\left[ \\text{Ag}\\left( {{\\text{S}}_{\\text{2}}}{{\\text{O}}_{\\text{3}}} \\right) \\right]}^{-}} \\right\\}$时,$\\textit{c}\\left( \\text{B}\\text{r}^{-}\\right)\\gt \\textit{c}\\left( \\text{S}_{\\text{2}}\\text{O}_{\\text{3}}^{\\text{2-}}\\right)\\gt \\textit{c}\\left\\{ \\left[ \\text{Ag}\\left( \\text{S}_{\\text{2}}\\text{O}_{\\text{3}}\\right)_{\\text{2}}\\right]^{\\text{3-}}\\right\\}\\gt \\textit{c}\\left( \\text{A}\\text{g}^{+}\\right)$
"]溴化银的饱和溶液中溴离子浓度和银离子浓度相等,向饱和溶液中滴加硫代硫酸钠溶液时,溶液中银离子浓度减小、溴离子浓度增大,溴化银与硫代硫酸钠溶液开始反应时,溴化银主要转化为$\rm [Ag(S_{2}O_{3})]^{-}$,故开始溶液中$\rm \dfrac{\textit{c}(A{{\text{g}}^{+}}\text{)}}{\textit{c }\!\!\{\ \!\![\!\!\text{ Ag(}{{\text{S}}_{2}}{{\text{O}}_{3}}\text{)}{{]}^{-}}\}}$小于$\rm \dfrac{\textit{c}(A{{\text{g}}^{+}}\text{)}}{\textit{c }\!\!\{\ \![\!\!\text{ Ag(}{{\text{S}}_{2}}{{\text{O}}_{3}}{{\text{)}}_{2}}{{]}^{3-}}\}}$。
$\rm A$.$K_\rm{sp}(AgBr)=10^{-12.2}$,则$ 10^{-3.85}\times 10^{x}=10^{-12.2}$,$ x=-8.35$,故$\rm A$正确;
$\rm B$.由图可知,溶液中硫代硫酸根离子浓度为$\rm 10^{-4.6}\;\rm mol/L$时,溶液中溴离子和银离子浓度分别为$\rm 10^{-3.85}\;\rm mol/L$、$\rm 10^{-8.35}\;\rm mol/L$,$\dfrac{c\left( \text{A}{{\text{g}}^{+}} \right)}{c\left\{ {{\left[ \text{Ag}{{\left( {{\text{S}}_{2}}{{\text{O}}_{3}} \right)}_{2}} \right]}^{3-}} \right\}}\rm =10^{-4.2}$,${{\left[ \text{Ag}{{\left( {{\text{S}}_{2}}{{\text{O}}_{3}} \right)}_{2}} \right]}^{3-}}$的浓度为:$\dfrac{\text{1}{{\text{0}}^{\text{-8}\text{.35}}}}{\text{1}{{\text{0}}^{\text{-4}\text{.2}}}}\ \text{mol/L=1}{{\text{0}}^{\text{-4}\text{.15}}}\ \text{mol/L}$,$\text{AgBr}+2{{\text{S}}_{2}}\text{O}_{3}^{2-}={{\left[ \text{Ag}{{\left( {{\text{S}}_{2}}{{\text{O}}_{3}} \right)}_{3}} \right]}^{3-}}+\text{B}{{\text{r}}^{-}}$的平衡常数为$\dfrac{{{10}^{-4.15}}\times {{10}^{-3.85}}}{{{({{10}^{-4.6}})}^{2}}}={{10}^{1.2}}$ ,故$\rm B$正确;
$\rm C$.根据物料守恒,($\rm -4.6$,$\rm -4.2$)点存在$ c\rm (Br^{-})$ $\rm =$ $ c\rm(Ag^{+})$ $\rm +$ $ c\rm{[Ag(S_{2}O_{3})]^{-}}$ $\rm +$ $ c\rm {[Ag(S_{2}O_{3})_{2}]^{3-}}$, $\dfrac{c(\text{B}{{\text{r}}^{-}})\times c{{\left[ \text{Ag}{{\left( {{\text{S}}_{2}}{{\text{O}}_{3}} \right)}_{2}} \right]}^{3-}}}{c{{({{\text{S}}_{2}}\text{O}_{3}^{2-})}^{2}}}=\dfrac{c(\text{B}{{\text{r}}^{-}})\times c{{\left[ \text{Ag}{{\left( {{\text{S}}_{2}}{{\text{O}}_{3}} \right)}_{2}} \right]}^{3-}}}{c{{({{\text{S}}_{2}}\text{O}_{3}^{2-})}^{2}}}={{10}^{1.2}}$,$\textit{c}\left( {{\text{S}}_{\text{2}}}\text{O}_{\text{3}}^{\text{2-}} \right)\textit{=c}\left\{ {{\left[ \text{Ag}{{\left( {{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}} \right)}_{\text{2}}} \right]}^{\text{3-}}} \right\}$时,$\left( \text{1}{{\text{0}}^{\text{1.2}}}-1 \right)\textit{c}\left( {{\text{S}}_{\text{2}}}\text{O}_{\text{3}}^{\text{2-}} \right)=\textit{c}(\text{B}{{\text{r}}^{-}})-\textit{c}({{\text{S}}_{2}}\text{O}_{3}^{2-})=\textit{c}\left( \text{A}{{\text{g}}^{+}} \right)+\textit{c}\left\{ {{\left[ \text{Ag}\left( {{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}} \right) \right]}^{-}} \right\}$,故$ c\rm(Br^{-})$ $\rm =$ $c\rm(Ag^{+})$ $\rm +$ $ c\rm{[Ag(S_{2}O_{3})]^{-}}$ $\rm +$ $ c\rm{[Ag(S_{2}O_{3})_{2}]^{3-}}$,故$\rm C$正确;
$\rm D$.溴化银与硫代硫酸钠溶液开始反应时,溴化银主要转化为${{[\text{Ag}({{\text{S}}_{2}}{{\text{O}}_{3}})]}^{-}}$,${{\text{S}}_{2}}\text{O}_{3}^{2-}$浓度逐渐减小,溶液中$\dfrac{c(\text{A}{{\text{g}}^{+}})}{c\{{{[\text{Ag}({{\text{S}}_{2}}{{\text{O}}_{3}})]}^{-}}\}}$小于$\dfrac{c(\text{A}{{\text{g}}^{+}})}{c\{{{[\text{Ag}{{({{\text{S}}_{2}}{{\text{O}}_{3}})}_{2}}]}^{3-}}\}}$,($\rm -4.6$,$\rm -4.2$)点存在$\dfrac{c(\text{A}{{\text{g}}^{+}})}{c\{{{[\text{Ag}{{({{\text{S}}_{2}}{{\text{O}}_{3}})}_{2}}]}^{3-}}\}}\rm =\dfrac{\textit{c}(\text{A}{{\text{g}}^{+}})}{\textit{c}\{{{[\text{Ag}({{\text{S}}_{2}}{{\text{O}}_{3}})]}^{-}}\}}$,该点左侧$c\rm{[Ag(S_{2}O_{3})]^{-}}$ $\rm \gt $ $ c\rm{[Ag(S_{2}O_{3})_{2}]^{3-}}$,而该点右侧$ c\rm{[Ag(S_{2}O_{3})_{2}]^{3-}}\gt \textit{c}{[Ag(S_{2}O_{3})]^{-}}$,$\textit{c}\left( {{\text{S}}_{\text{2}}}\text{O}_{\text{3}}^{\text{2-}} \right)=\textit{c}\left\{ {{\left[ \text{Ag}\left( {{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}} \right) \right]}^{-}} \right\}$时,$\text{N}\text{a}_{2}\text{S}_{2}\text{O}_{3}$固体完全溶解,位于该点右侧,结合分析,存在$\textit{c}\left( \text{B}\text{r}^{-}\right)\gt \textit{c}\left\{ \left[ \text{Ag}\left( \text{S}_{\text{2}}\text{O}_{\text{3}}\right)_{\text{2}}\right]^{\text{3-}}\right\}\gt \textit{c}\left( \text{S}_{\text{2}}\text{O}_{\text{3}}^{\text{2-}}\right)\gt \textit{c}\left( \text{A}\text{g}^{+}\right)$,故$\rm D$错误;
故选:$\rm D$
高中 | 盐溶液微粒间的三大守恒原理的理解及应用题目答案及解析(完整版)
