高中 | 化学平衡常数 题目答案及解析
稿件来源:高途
高中 | 化学平衡常数题目答案及解析如下,仅供参考!
选修四
第二章 化学反应速率和化学平衡
第三节 化学平衡
化学平衡常数
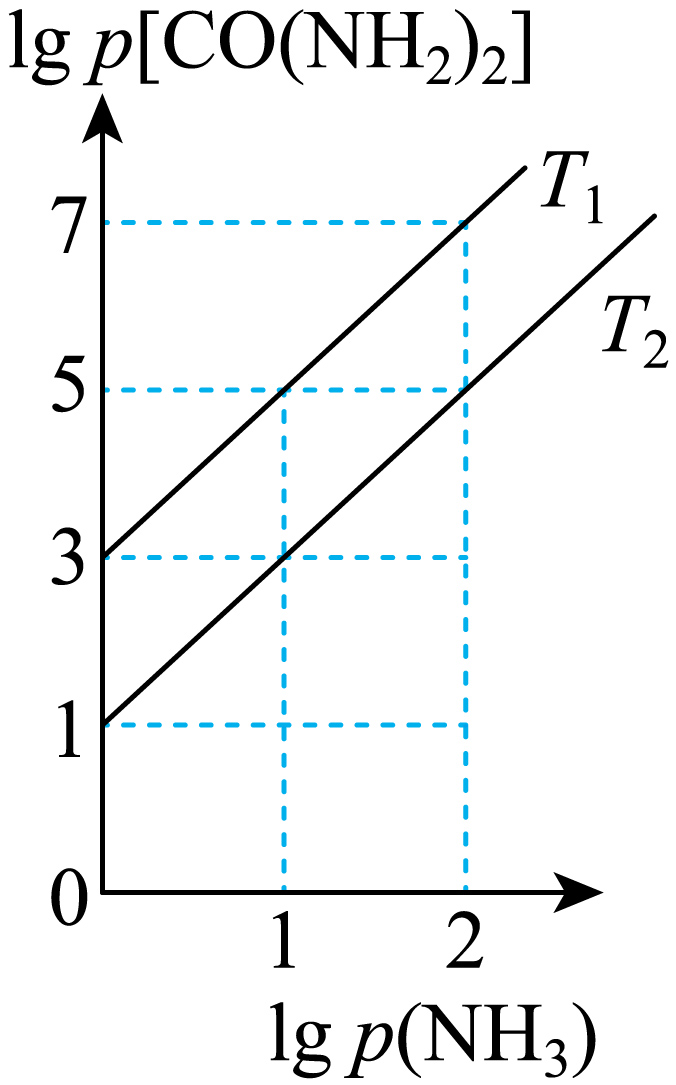
工业合成尿素:$\text{HNCO}\left( \text{g} \right)\text{+N}{{\text{H}}_{\text{3}}}\left( \,\text{g} \right)\rightleftharpoons \text{CO}{{\left( \text{N}{{\text{H}}_{\text{2}}} \right)}_{\text{2}}}\left( \,\text{g} \right)\qquad\Delta\!\!\textit{ H}\lt 0$。恒容密闭容器加入等物质的量的$\text{HNCO}$和$\text{N}{{\text{H}}_{3}}$,在$\textit{T}_{1}$和$\textit{T}_{2}$反应达到平衡,$\text{lg}p\left( \text{N}\text{H}_{\text{3}}\right)$与$\text{lg}p\left[ \text{CO}\left( \text{N}\text{H}_{\text{2}}\right)_{\text{2}}\right]$关系如图所示$\rm ($压强单位均为$\text{kPa}\rm )$。反应$d\text{D}\left( \text{g}\right)+e\text{E}\left( \text{g}\right)\rightleftharpoons g\text{G}\left( \text{g}\right)$的标准平衡常数$\textit{K}^{\textit{o}}=\dfrac{\left[ \dfrac{\textit{p}\left( \text{G}\right)}{\textit{p}^{\textit{o}}}\right]^{\textit{g}}}{\left[ \dfrac{\textit{p}\left( \text{D}\right)}{\textit{p}^{\textit{o}}}\right]^{\textit{d}}\left[ \dfrac{\textit{p}\left( \text{E}\right)}{\textit{p}^{\textit{o}}}\right]^{\textit{e}}},\textit{p}^{^{o}}=100\ \text{kPa}$。下列说法正确的是$(\qquad)$
$\\textit{T}_{\\text{1}}\\gt \\textit{T}_{\\text{2}}$
","$\\dfrac{\\textit{K}_{\\text{p}}\\left( \\textit{T}_{\\text{1}}\\right)}{\\textit{K}_{\\text{p}}\\left( \\textit{T}_{\\text{2}}\\right)}=\\dfrac{\\textit{K}^{\\text{o}}\\left( \\textit{T}_{\\text{1}}\\right)}{\\textit{K}^{\\text{o}}\\left( \\textit{T}_{\\text{2}}\\right)}$
","$\\textit{T}_{2}$下,平衡时体系的总压强为$250\\;\\rm \\text{kPa}$时,$\\textit{p}\\left( \\text{N}{{\\text{H}}_{\\text{3}}} \\right)\\approx 4.9\\;\\rm \\text{kPa}$
","若改为恒压密闭容器,则平衡时$\\text{CO}{{\\left( \\text{N}{{\\text{H}}_{2}} \\right)}_{2}}$的体积分数减小
"]$\textit{p}\left( \text{N}\text{H}_{\text{3}}\right)$表示物质$\text{N}{{\text{H}}_{\text{3}}}$的分压强,$\text{lg}p\left( \text{N}\text{H}_{\text{3}}\right)$值越大,说明其分压越大,说明其体积分数或物质的量分数越大,据此分析解答。
$\rm A$.平衡时,相同的$\text{lg}p\left( \text{N}\text{H}_{\text{3}}\right)$时,温度${{\textit{T}}_{2}}$时的$\text{lg}p\left[ \text{CO}{{\left( \text{N}{{\text{H}}_{\text{2}}} \right)}_{\text{2}}} \right]$小于${{\textit{T}}_{1}}$时的$\text{lg}p\left[ \text{CO}{{\left( \text{N}{{\text{H}}_{\text{2}}} \right)}_{\text{2}}} \right]$ ,说明温度${{\textit{T}}_{1}}$到${{\textit{T}}_{2}\;\rm ^\circ\rm C}$时,平衡逆向移动,该反应是放热反应,则$\textit{T}_{\text{1}}\lt \textit{T}_{\text{2}}$,故$\rm A$错误;
$\rm B$.$\textit{K}^{\text{o}}=\dfrac{\left[ \dfrac{\textit{p}\left( \text{G}\right)}{\textit{p}^{\textit{o}}}\right]^{\textit{g}}}{\left[ \dfrac{\textit{p}\left( \text{D}\right)}{\textit{p}^{\textit{o}}}\right]^{\textit{d}}\left[ \dfrac{\textit{p}\left( \text{E}\right)}{\textit{p}^{\textit{o}}}\right]^{^{\textit{e}}}}=\dfrac{\textit{p}\left( \text{G}\right)^{\textit{g}}}{\textit{p}\left( \text{D}\right)^{\textit{d}}\textit{p}\left( \text{E}\right)^{\textit{e}}}\times\left[ \textit{p}^{\textit{o}}\right]^{\textit{d}+\textit{e}-\textit{g}}=\textit{K}_\rm{p}\left[ \textit{p}^{\textit{o}}\right]^{\textit{d}+\textit{e}-\textit{g}}$,则${{\textit{K}}^{\textit{o}}}\left( {{\textit{T}}_{\text{1}}} \right)\rm ={{\textit{K}}_{\text{p}}}\left( {{\textit{T}}_{\text{1}}} \right){{\left[ {{\textit{p}}^{\textit{o}}} \right]}^{\textit{d}+\textit{e}-\textit{g}}}$,${{\textit{K}}^{\text{o}}}\left( {{\textit{T}}_{\text{2}}} \right)\rm ={{\textit{K}}_{\text{p}}}\left( {{\textit{T}}_{\text{2}}} \right){{\left[ {{\textit{p}}^{\textit{o}}} \right]}^{\textit{d}+\textit{e}-\textit{g}}}$,故$\dfrac{\textit{K}_{\text{p}}\left( \textit{T}_{\text{1}}\right)}{\textit{K}_{\text{p}}\left( \textit{T}_{\text{2}}\right)}=\dfrac{\textit{K}^{\textit{o}}\left( \textit{T}_{\text{1}}\right)}{\textit{K}^{\textit{o}}\left( \textit{T}_{\text{2}}\right)}$,故$\rm B$正确;
$\rm C$.$\textit{T}_{2}$时,$\text{lg}p\left( \text{N}{{\text{H}}_{\text{3}}} \right)\rm =1$,$\text{lg}p\left[ \text{CO}{{\left( \text{N}{{\text{H}}_{\text{2}}} \right)}_{\text{2}}} \right]\rm =3$,则$\textit{p}\left( \text{N}{{\text{H}}_{\text{3}}} \right)\rm =10$,$\textit{p}\left( \text{HNCO}\right)=10$,$\textit{p}\left( \text{CO}{{\left( \text{N}{{\text{H}}_{\text{2}}} \right)}_{\text{2}}} \right)\rm =1000$,则$\textit{K}_\rm{p}\left( \textit{T}_{2}\right)=\dfrac{\textit{p}\left( \text{CO}\left( \text{N}\text{H}_{\text{2}}\right)_{\text{2}}\right)}{\textit{p}\left( \text{N}\text{H}_{\text{3}}\right)\textit{p}\left( \text{HNCO}\right)}=\dfrac{1000}{100}=10$,若平衡时,$\textit{p}\left( \text{N}{{\text{H}}_{\text{3}}} \right)\approx 4.9\;\rm \text{kPa}$,则$\textit{K}_\rm{p}\left(\textit{T}_{2}\right)=\dfrac{\textit{p}\left( \text{CO}\left( \text{N}\text{H}_{\text{2}}\right)_{\text{2}}\right)}{\textit{p}\left( \text{N}\text{H}_{\text{3}}\right)\textit{p}\left( \text{HNCO}\right)}=\dfrac{\textit{p}\left( \text{CO}\left( \text{N}\text{H}_{\text{2}}\right)_{\text{2}}\right)}{4.9\times4.9}=10$,则$\textit{p}\left( \text{CO}{{\left( \text{N}{{\text{H}}_{\text{2}}} \right)}_{\text{2}}} \right)\rm =240.1$ $\text{kPa}$,${{\textit{P}}_{总}}=\textit{p}\left( \text{CO}{{\left( \text{N}{{\text{H}}_{\text{2}}} \right)}_{\text{2}}} \right)+\textit{p}\left( \text{N}{{\text{H}}_{\text{3}}} \right)+\textit{p}\left( \text{HNCO} \right)=240.1+4.9+4.9=249.9\approx 250\ \text{kPa}$ ,则平衡时体系的总压强为$250\;\rm \text{kPa}$,故$\rm C$正确;
$\rm D$.此反应$\text{HNCO}\left( \text{g} \right)\text{+N}{{\text{H}}_{\text{3}}}\left( \ \text{g} \right)\rightleftharpoons \text{CO}{{\left( \text{N}{{\text{H}}_{\text{2}}} \right)}_{\text{2}}}\left( \ \text{g} \right)\ $是总物质的量减小的反应,若改为恒压密闭容器,相对于恒容密闭容器来说,相当于增大压强的反应,增大压强,平衡会正向移动,则若改为恒压密闭容器,平衡时$\text{CO}{{\left( \text{N}{{\text{H}}_{2}} \right)}_{2}}$的体积分数会增大,故$\rm D$错误;
故选:$\rm BC$
高中 | 化学平衡常数题目答案及解析(完整版)
