| 盐溶液微粒间的三大守恒原理的理解及应用 题目答案及解析
稿件来源:高途
| 盐溶液微粒间的三大守恒原理的理解及应用题目答案及解析如下,仅供参考!
选修四
第三章 水溶液中的离子平衡
第三节 盐类的水解
盐溶液微粒间的三大守恒原理的理解及应用
$\text{F}{{\text{e}}^{3+}}$会与溶液中的$\text{C}{{\text{l}}^{-}}$形成多种配合物,${c}\left( \text{C}{{\text{l}}^{-}} \right)\lt 1\;\rm \text{mol}/\text{L}$时,溶液中相关微粒主要有如下$\rm 2$个平衡:
$\text{F}{{\text{e}}^{3+}}+\text{C}{{\text{l}}^{-}}\rightleftharpoons \text{FeC}{{\text{l}}^{2+}}\quad {{{K}}_{1}}={{10}^{1.8}}$
$\text{FeC}{{\text{l}}^{2+}}+\text{C}{{\text{l}}^{-}}\rightleftharpoons \text{FeC}{\text{l}}_{2}^{+}\quad {{{K}}_{2}}={{10}^{0.6}}$
改变$\text{C}{{\text{l}}^{-}}$的起始浓度,测得平衡时$\text{F}{{\text{e}}^{3+}}$、$\text{FeC}{{\text{l}}^{2+}}$、$\mathrm{FeCl}_2^{+}$的分布系数$\delta$随$\lg \left( \text{C}{{\text{l}}^{-}} \right)$的变化如图所示(部分图)。已知:$\delta\left( \text{F}{{\text{e}}^{3+}} \right)=\dfrac{{c}\left( \text{F}{{\text{e}}^{3+}} \right)}{{c}\left( \text{F}{{\text{e}}^{3+}} \right)+{c}\left( \text{FeC}{{\text{l}}^{2+}} \right)+{c}\left( \text{FeCl}_{2}^{+} \right)}$,${{10}^{0.6}}\approx 4$。下列说法正确的是$(\qquad)$
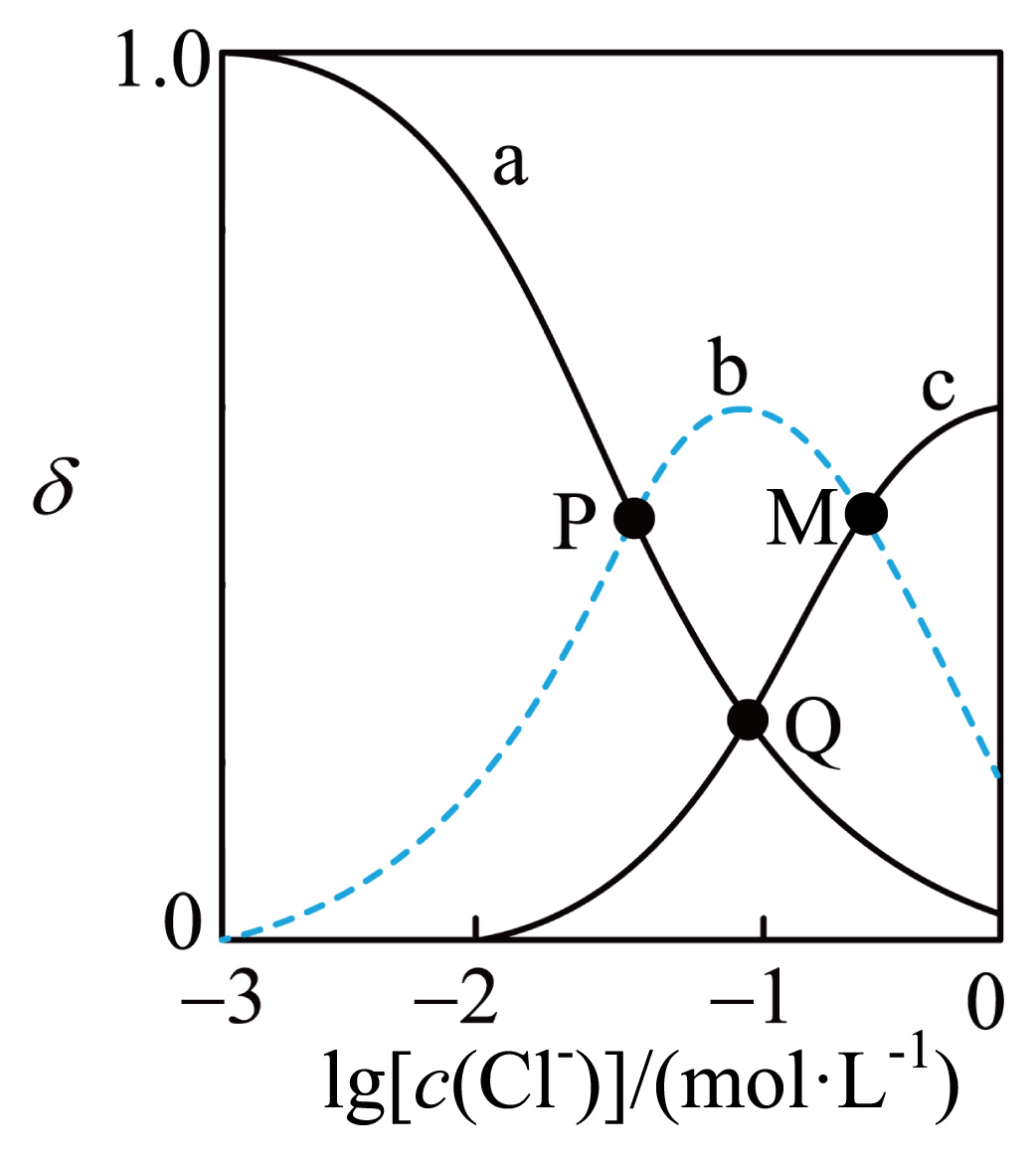
曲线$\\rm b$表示的微粒是$\\mathrm{FeCl}_2^{+}$
","$\\rm Q$点溶液中${c}\\left( \\text{C}{{\\text{l}}^{-}} \\right)={{10}^{-0.6}}\\;\\rm \\text{mol}/\\text{L}$
","$\\rm Q$点溶液中$\\text{F}{{\\text{e}}^{3+}}$的转化率约为$\\rm 83\\%$
","$\\rm M$点溶液中一定存在:$3{c}\\left( \\text{F}{{\\text{e}}^{3+}} \\right)+3{c}\\left( \\text{FeC}{{\\text{l}}^{2+}} \\right)+{c}\\left( {{\\text{H}}^{+}} \\right)={c}\\left( \\text{O}{{\\text{H}}^{-}} \\right)+{c}\\left( \\text{C}{{\\text{l}}^{-}} \\right)$
"]$c(\rm Fe^{3+})$随$c(\rm Cl^{-})$的上升而下降,$ c(\rm FeCl^{2+})$随$c(\rm Cl^{-})$的上升先升后降,$\rm c(\rm {FeCl}_2^{+}\rm )$随$c(\rm Cl^{-})$的上升而上升,即曲线$\rm a$为$\rm Fe^{3+}$,曲线$\rm b$为$\rm FeCl^{2+}$,曲线$\rm c$为$\mathrm{FeCl}_2^{+}$,据此分析;
$\rm A$.随着$\text{C}{{\text{l}}^{-}}$的起始浓度增大,依次生成$\text{FeC}{{\text{l}}^{2+}}$和$\text{FeCl}_{2}^{+}$,故曲线$\rm a$、$\rm b$、$\rm c$分别表示$\text{F}{{\text{e}}^{3+}}、\text{FeC}{{\text{l}}^{2+}}、\text{FeCl}_{2}^{+}$,$\rm A$错误;
$\rm B$.$\rm Q$点溶液,${c}\left( \text{F}{{\text{e}}^{3+}} \right)={c}\left( \text{FeCl}_{2}^{+} \right)$,${{{K}}_{1}}{{{K}}_{2}}=\dfrac{{c}\left( \text{FeC}{{\text{l}}^{2+}} \right){c}\left( \text{FeCl}_{2}^{+} \right)}{{c}\left( \text{F}{{\text{e}}^{3+}} \right){c}\left( \text{C}{{\text{l}}^{-}} \right){c}\left( \text{FeC}{{\text{l}}^{2+}} \right){c}\left( \text{C}{{\text{l}}^{-}} \right)}=\dfrac{{c}\left( \text{FeCl}_{2}^{+} \right)}{{c}\left( \text{F}{{\text{e}}^{3+}} \right){{{c}}^{2}}\left( \text{C}{{\text{l}}^{-}} \right)}=\dfrac{1}{{{\text{c}}^{2}}\left( \text{C}{{\text{l}}^{-}} \right)}={{10}^{2,4}}$得${c}\left( \text{C}{{\text{l}}^{-}} \right)={{10}^{-1.2}}\;\rm \text{mol}/\text{L}$,$\rm B$错误;
$\rm C$.设$\rm Q$点溶液${c}\left( \text{F}{{\text{e}}^{3+}} \right)={c}\left( \text{FeCl}_{2}^{+} \right)={x\ \rm mol}/\text{L}$,据${{{K}}_{1}}=\dfrac{{c}\left( \text{FeC}{{\text{l}}^{2+}} \right)}{{c}\left( \text{F}{{\text{e}}^{3+}} \right){c}\left( \text{C}{{\text{l}}^{-}} \right)}={{10}^{1.8}}$,得${c}\left( \text{FeC}{{\text{l}}^{2+}} \right)={{10}^{1.8}}{c}\left( \text{F}{{\text{e}}^{3+}} \right){c}\left( \text{C}{{\text{l}}^{-}} \right)={{10}^{1.8}}\times {x}\times {{10}^{-1.2}}={{10}^{0.6}}{x}\left( \text{mol}/\text{L} \right)$,$\text{F}{{\text{e}}^{3+}}$的转化率$=\dfrac{{c}\left( \text{FeC}{{\text{l}}^{2+}} \right)+{c}\left( \text{FeCl}_{2}^{+} \right)}{{c}\left( \text{F}{{\text{e}}^{3+}} \right)+{c}\left( \text{FeC}{{\text{l}}^{2+}} \right)+{c}\left( \text{FeCl}_{2}^{+} \right)}\times 100\%=\dfrac{{{10}^{0.6}}{x}+{x}}{{x}+{{10}^{0.6}}{x}+{x}}\times 100\%=\dfrac{5}{6}\times 100\%=83\%$,$\rm C$正确;
$\rm D$.$\rm M$点溶液${c}\left( \text{FeC}{{\text{l}}^{2+}} \right)={c}\left( \text{FeCl}_{2}^{+} \right)$,由电荷守恒可知,${3c}\left( \text{F}{{\text{e}}^{\text{3+}}} \right){+2c}\left( \text{FeC}{{\text{l}}^{\text{2+}}} \right){+c}\left( {{\text{H}}^{+}} \right){+c}\left( \text{FeCl}_{\text{2}}^{+} \right){=c}\left( \text{O}{{\text{H}}^{-}} \right){+c}\left( \text{C}{{\text{l}}^{-}} \right)$,可知,$3{c}\left( \text{F}{{\text{e}}^{3+}} \right)+3{c}\left( \text{FeC}{{\text{l}}^{2+}} \right)+{c}\left( {{\text{H}}^{+}} \right)={c}\left( \text{O}{{\text{H}}^{-}} \right)+{c}\left( \text{C}{{\text{l}}^{-}} \right)$,因$\text{F}{{\text{e}}^{3+}}$会与溶液中的$\text{C}{{\text{l}}^{-}}$形成多种配合物,可能会有其他离子,$\rm D$项关系式不一定正确,$\rm D$错误;
故选:$\rm C$
| 盐溶液微粒间的三大守恒原理的理解及应用题目答案及解析(完整版)
