高中 | 化学平衡常数 题目答案及解析
稿件来源:高途
高中 | 化学平衡常数题目答案及解析如下,仅供参考!
选修四
第二章 化学反应速率和化学平衡
第三节 化学平衡
化学平衡常数
利用工业废气中的$\text{C}{{\text{O}}_{2}}$合成甲醇可以变废为宝,有关反应式如下:
$\rm i$.$\text{C}{{\text{O}}_{2}}(\text{g})+3{{\text{H}}_{2}}(\text{g})\rightleftharpoons \text{C}{{\text{H}}_{3}}\text{OH}(\text{g})+{{\text{H}}_{2}}\text{O}(\text{g})$
$\rm ii$.$\text{C}{{\text{O}}_{2}}(\text{g})+{{\text{H}}_{2}}(\text{g})\rightleftharpoons \text{CO}(\text{g})+{{\text{H}}_{2}}\text{O}(\text{g})$
上述反应的焓变和熵变如表:
| 反应 | $\Delta H/\left( \text{kJ}\cdot \text{mo}{{\text{l}}^{-1}} \right)$ | $\Delta S/\left( \text{J}\cdot \text{mo}{{\text{l}}^{-1}}\cdot {{\text{K}}^{-1}} \right)$ |
| $\rm i$ | $-48.97$ | $-177.76$ |
| $\rm ii$ | $+41.17$ | $+42.08$ |
回答下列问题:
该过程使用的催化剂为$\text{Cu}/\text{Zn}/\text{Al}$纳米纤维,该催化剂中过渡元素第二电离能与第一电离能相差最小的是 $\rm ($填元素符号$\rm )$。
$\\rm Zn$
"]]$\rm Al$不是过渡元素,基态$\rm \text{Zn}$的价电子排布式为$\rm 3 d^{10} 4 s^{2}$,基态$\rm \text{Z}{{\text{n}}^{+}}$的价电子排布式为$\rm \text{3}{{\text{d}}^{\text{10}}}\text{4}{{\text{s}}^{\text{1}}}$,基态$\rm \text{Cu}$价电子排布式为$\rm \text{3}{{\text{d}}^{\text{10}}}\text{4}{{\text{s}}^{\text{1}}}$,基态$\rm \text{C}{{\text{u}}^{+}}$价电子排布式为$\rm 3 d^{10}$,处于半满状态,难失去电子,其第二电离能大,第二电离能与第一电离能相差大,故该催化剂中过渡元素第二电离能与第一电离能相差最小的是$\rm Zn$;
反应$\rm i$正反应自发进行的条件是 $\rm ($填“低温”“高温”或“任意温度”$\rm )$。
低温
"]]反应$\rm i$的$\Delta H\lt 0$、$\Delta S\lt 0$,依据$\Delta G=\Delta H-T\Delta S\lt 0$反应可自发进行,则低温下自发进行;
已知:①$\text{C}{{\text{H}}_{3}}\text{OH}(\text{l})+\dfrac{3}{2}{{\text{O}}_{2}}(\text{g})=\text{C}{{\text{O}}_{2}}(\text{g})+2{{\text{H}}_{2}}\text{O(l)}\quad \Delta {{H}_{1}}=-726.51\,\text{kJ}\cdot \text{mo}{{\text{l}}^{-1}}$
②${{\text{H}}_{2}}(\text{g})+\dfrac{1}{2}{{\text{O}}_{2}}(\text{g})={{\text{H}}_{2}}\text{O}(\text{l})\quad \Delta {{H}_{2}}$
③${{\text{H}}_{2}}\text{O}(\text{g})={{\text{H}}_{2}}\text{O}(\text{l})\quad \Delta {{H}_{3}}=-44\,\text{kJ}\cdot \text{mo}{{\text{l}}^{-1}}$
④$\text{C}{{\text{H}}_{3}}\text{OH}(\text{l})\rightleftharpoons \text{C}{{\text{H}}_{3}}\text{OH}(\text{g})\quad \Delta {{H}_{4}}=+37.92\,\text{kJ}\cdot \text{mo}{{\text{l}}^{-1}}$则$\Delta {{H}_{2}}=$ $\;\rm \text{kJ}\cdot \text{mo}{{\text{l}}^{-1}}$。
$\\rm -285.8$
"]]已知:①$\text{C}{{\text{H}}_{3}}\text{OH}(\text{l})+\dfrac{3}{2}{{\text{O}}_{2}}(\text{g})=\text{C}{{\text{O}}_{2}}(\text{g})+2{{\text{H}}_{2}}\text{O(l)}\quad \Delta {{H}_{1}}=-726.51\,\text{kJ}\cdot \text{mo}{{\text{l}}^{-1}}$,②${{\text{H}}_{2}}(\text{g})+\dfrac{1}{2}{{\text{O}}_{2}}(\text{g})={{\text{H}}_{2}}\text{O}(\text{l})\quad \Delta {{H}_{2}}$,③${{\text{H}}_{2}}\text{O}(\text{g})={{\text{H}}_{2}}\text{O}(\text{l})\quad \Delta {{H}_{3}}=-44\,\text{kJ}\cdot \text{mo}{{\text{l}}^{-1}}$,④$\text{C}{{\text{H}}_{3}}\text{OH}(\text{l})\rightleftharpoons \text{C}{{\text{H}}_{3}}\text{OH}(\text{g})\quad \Delta {{H}_{4}}=+37.92\,\text{kJ}\cdot \text{mo}{{\text{l}}^{-1}}$;⑤$\text{C}{{\text{O}}_{\text{2}}}\left( \text{g} \right)\text{+3}{{\text{H}}_{\text{2}}}\left( \text{g} \right)\rightleftharpoons \text{C}{{\text{H}}_{\text{3}}}\text{OH}\left( \text{g} \right)+{{\text{H}}_{\text{2}}}\text{O}\left( \text{g} \right)\quad \Delta {{H}_{\text{5}}}=-\text{48}.97\,\text{kJ}\cdot \text{mo}{{\text{l}}^{-1}}$,根据盖斯定律:①$\rm +$③$\rm -$④$\rm +$⑤得到$\text{3}{{\text{H}}_{\text{2}}}\left( \text{g} \right)+\dfrac{\text{3}}{\text{2}}{{\text{O}}_{\text{2}}}\left( \text{g} \right)\text{=3}{{\text{H}}_{\text{2}}}\text{O}\left( \text{l} \right)$,求$\Delta H=-726.51\,\text{kJ}\cdot \text{mo}{{\text{l}}^{-1}}+\left( -44\,\text{kJ}\cdot \text{mo}{{\text{l}}^{-1}} \right)-\left( +37.92\,\text{kJ}\cdot \text{mo}{{\text{l}}^{-1}} \right)+\left( -48.97\,\text{kJ}\cdot \text{mo}{{\text{l}}^{-1}} \right)=\text{-857}.4\,\text{kJ}\cdot \text{mo}{{\text{l}}^{-1}}$;则${{\text{H}}_{\text{2}}}\left( \text{g} \right)+\dfrac{\text{1}}{\text{2}}{{\text{O}}_{\text{2}}}\left( \text{g} \right)={{\text{H}}_{\text{2}}}\text{O}\left( \text{l} \right)\quad \Delta {{H}_{\text{2}}}=\dfrac{-857.4\,\text{kJ}\cdot \text{mo}{{\text{l}}^{-1}}}{3}=-285.8\,\text{kJ}\cdot \text{mo}{{\text{l}}^{-1}}$;
在恒容密闭容器中充入$1\text{ mol C}{{\text{O}}_{2}}$和$3\,\text{mol}\,{{\text{H}}_{2}}$,发生上述反应合成$\text{C}{{\text{H}}_{3}}\text{OH}$,测得$\text{C}{{\text{H}}_{3}}\text{OH}$的选择性和$\text{C}{{\text{O}}_{2}}$平衡转化率与温度的关系如图所示。
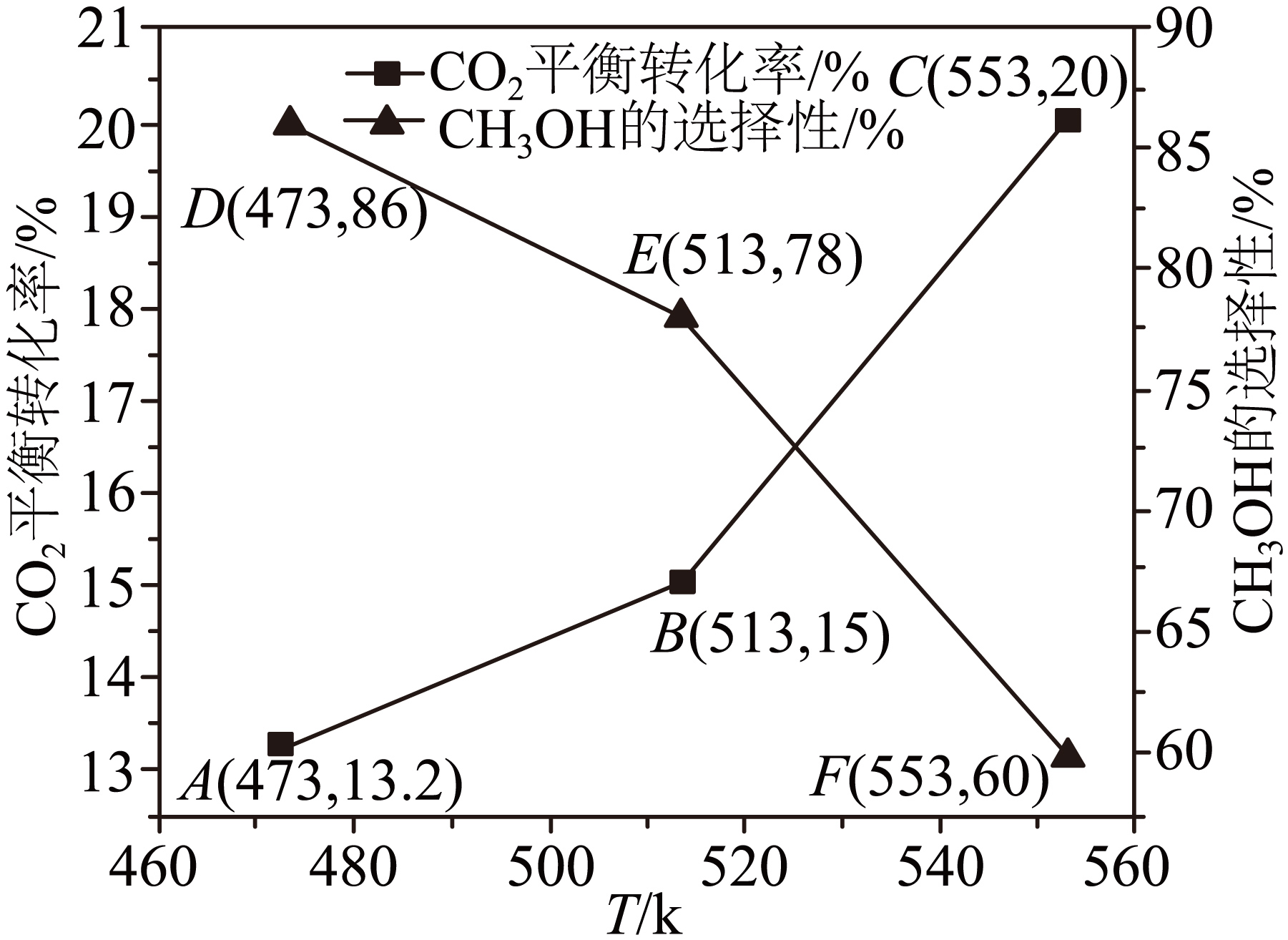
$\rm D$、$\rm E$、$\rm F$三点中,$\text{C}{{\text{H}}_{3}}\text{OH}$的物质的量最大的是 $\rm ($填字母$\rm )$。温度高于$\text{513 K}$时,$\text{C}{{\text{O}}_{2}}$平衡转化率增大程度加快,其主要原因是 。
提示:$\text{C}{{\text{H}}_{3}}\text{OH}的选择性=\dfrac{n\left( \text{C}{{\text{H}}_{3}}\text{OH} \right)}{n\left( \text{C}{{\text{H}}_{3}}\text{OH} \right)+n(\text{CO})}\times 100\%$。
$\\rm F$ ; 随着温度升高,反应ⅱ向右移动的程度大于反应ⅰ向左移动的程度
"]]$\text{D}$、$\text{E}$、$\text{F}$点生成$\text{C}{{\text{H}}_{3}}\text{OH}$的物质的量分别为$n(\text{D})=1\,\text{mol}\times 13.2\%\times 86\%\approx 0.1135\,\text{mol}$、$n(\text{E})=1\,\text{mol}\times 15\%\times 78\%=0.117\,\text{mol}$、$n(\text{F})=1\,\text{mol}\times 20\%\times 60\%=0.12\,\text{mol}$,$\text{C}{{\text{H}}_{3}}\text{OH}$的物质的量最大的是$\rm F$点;升高温度反应$\rm i$平衡左移,二氧化碳平衡转化率降低,而反应ⅱ平衡右移,二氧化碳平衡转化率增大,温度高于$\text{513 K}$时,$\text{C}{{\text{O}}_{\text{2}}}$平衡转化率增大程度加快,说明此时以反应ⅱ为主,即随着温度升高,反应ⅱ向右移动的程度大于反应ⅰ向左移动的程度;
某温度下,保持总压强为$\text{100 kPa}$,向体积可变的密闭容器中充入$\text{1 mol C}{{\text{O}}_{\text{2}}}$和$\text{3 mol }{{\text{H}}_{2}}$,发生反应,达到平衡时$\text{C}{{\text{O}}_{2}}$转化率为$50\%$,$\text{C}{{\text{H}}_{3}}\text{OH}$体积分数为$10\%$。平衡体系中,${{\text{H}}_{2}}$分压为 $\text{kPa}$。该温度下,反应$\rm i$的平衡常数${{K}_{\text{p}}}$为 $\rm ($列出计算式$\rm ){{(\text{kPa})}^{-2}}$。
提示:用分压代替浓度计算的平衡常数叫压强平衡常数$\left( {{K}_{\text{p}}} \right)$,分压等于总压$\rm \times $物质的量分数。
$\\rm 55$ ; $\\dfrac{10\\times 15}{15\\times {{55}^{3}}}$或$\\dfrac{10}{{{55}^{3}}}$
"]]设平衡体系中,$\text{C}{{\text{H}}_{3}}\text{OH}、\text{CO}$的物质的量分别为$x$、$y$,列式子:$\rm i$.$\begin{matrix} {} & \text{C}{{\text{O}}_{\text{2}}}\text{(g)} & + & \text{3}{{\text{H}}_{\text{2}}}\text{(g)} & \rightleftharpoons & \text{C}{{\text{H}}_{\text{3}}}\text{OH(g)} & + & {{\text{H}}_{\text{2}}}\text{O(g)} \\起始 (\text{mol}) & \text{1} & {} & \text{3} & {} & \text{0} & {} & \text{0} \\ 转化(\text{mol}) & x & {} & 3x & {} & x & {} & x \\ 平衡(\text{mol}) & 1-x-y & {} & 3-3x-y & {} & x & {} & x+y \\ \end{matrix}$,$\rm ii$.$\begin{matrix} {} & \text{C}{{\text{O}}_{\text{2}}}\text{(g)} & + & {{\text{H}}_{\text{2}}}\text{(g)} & \rightleftharpoons & \text{CO(g)} & + & {{\text{H}}_{\text{2}}}\text{O(g)} \\起始 (\text{mol}) & \text{1} & {} & \text{3} & {} & \text{0} & {} & \text{0} \\ 转化(\text{mol}) & y & {} & y & {} & y & {} & y \\平衡 (\text{mol}) & 1-x-y & {} & 3-3x-y & {} & y & {} & x+y \\ \end{matrix}$,依题意,$x+y=\text{0}\text{.5}\,\text{mol}$,$\dfrac{x}{4\,\text{mol}-2x}\text{=10 }\!\!\%$,解得:$x=\dfrac{\text{1}}{\text{3}}\,\text{mol}$,$y=\dfrac{\text{1}}{\text{6}}\,\text{mol}$;平衡体系中,各组分的物质的量如下:$\text{0}{.5\;\rm mol\;\rm C}{{\text{O}}_{\text{2}}}、\dfrac{\text{1}}{\text{3}}\ \text{mol\ C}{{\text{H}}_{\text{3}}}\text{OH}、\dfrac{\text{1}}{\text{6}}\ \text{mol\ CO}、\text{0}{.5\;\rm mol}\ {{\text{H}}_{2}}\text{O}、\dfrac{11}{6}\ \text{mol}\ {{\text{H}}_{2}}$,总物质的量$n=\left( 0.5+0.5+\dfrac{1}{3}+\dfrac{1}{6}+\dfrac{11}{6} \right)\ \text{ mol}=\dfrac{10}{3}\text{ mol}$,$p\left( \text{C}{{\text{O}}_{2}} \right)=100\ \text{ kPa}\times 0.5\ \text{ mol}\times \dfrac{3}{10\text{ mol}}=15\text{ kPa}$,$p(\text{CO})=5\,\text{kPa}$,$p\left( \text{C}{{\text{H}}_{3}}\text{OH} \right)=10\,\text{kPa}$,$p\left( {{\text{H}}_{2}} \right)=55\,\text{kPa}$,$p\left( {{\text{H}}_{2}}\text{O} \right)=15\,\text{kPa}$。反应$\rm i$的平衡常数${{K}_{\text{p}}}\text{(i)}=\dfrac{p\left( \text{C}{{\text{H}}_{3}}\text{OH} \right)\cdot p\left( {{\text{H}}_{2}}\text{O} \right)}{p\left( \text{C}{{\text{O}}_{2}} \right)\cdot {{p}^{3}}\left( {{\text{H}}_{2}} \right)}=\dfrac{10\,\text{kPa}\times 15\,\text{kPa}}{15\,\text{kPa}\times {{(55\,\text{kPa})}^{3}}}=\dfrac{10}{{{55}^{3}}}{{(\text{kPa})}^{-2}}$;
以$\text{C}{{\text{H}}_{3}}\text{OH}$为燃料制成碱性燃料$\left( \text{C}{{\text{H}}_{3}}\text{OH}/{{\text{O}}_{2}} \right)$电池,利用该电池电解$\text{500}\,\text{mL }1.0\text{ mol}\cdot {{\text{L}}^{-1}}\,\text{NaCl}$溶液,当正极消耗$\text{280}\,\text{mL}\rm ($标准状况$\rm )$纯气体时,电解装置恢复至室温,此时电解后的$\text{NaCl}$溶液的$\text{pH}$为 $\rm ($忽略溶液体积的变化$\rm )$。
$\\rm 13$
"]]正极的电极反应式为${{\text{O}}_{2}}+2{{\text{H}}_{2}}\text{O}+4{{\text{e}}^{-}}=4\text{O}{{\text{H}}^{-}}$,电解氯化钠溶液时阴极的电极反应式为$2{{\text{H}}_{2}}\text{O}+2{{\text{e}}^{-}}=2\text{O}{{\text{H}}^{-}}+{{\text{H}}_{2}}\uparrow $,转移电子的物质的量$n\left( {{\text{e}}^{-}} \right)=\dfrac{280\times {{10}^{-3}}\text{ L}}{22.4\text{ L}\cdot \text{mo}{{\text{l}}^{-1}}}\times 4=0.05\text{ mol}$,电解生成的氢氧根离子浓度$c\left( \text{O}{{\text{H}}^{-}} \right)=\dfrac{0.05\text{ mol}}{0.5\text{ L}}=0.1\text{ mol}\cdot {{\text{L}}^{-1}}$,$\text{pH}=14-p\text{OH}=13$。
高中 | 化学平衡常数题目答案及解析(完整版)
