高中 | 盐溶液微粒间的三大守恒原理的理解及应用 题目答案及解析
稿件来源:高途
高中 | 盐溶液微粒间的三大守恒原理的理解及应用题目答案及解析如下,仅供参考!
选修四
第三章 水溶液中的离子平衡
第三节 盐类的水解
盐溶液微粒间的三大守恒原理的理解及应用
$\text{C}{{\text{O}}_{2}}$资源化利用能有效减少$\text{C}{{\text{O}}_{2}}$排放,对低碳循环经济的发展具有重要意义。室温下以$0.1\ \text{mol}\cdot {{\text{L}}^{-1}}$的$\text{NaOH}$溶液吸收捕集烟气中的$\text{C}{{\text{O}}_{2}}$。通入$\text{C}{{\text{O}}_{2}}$引起的溶液体积变化和${{\text{H}}_{2}}\text{O}\left( \text{g} \right)$的挥发忽略不计,已知:${{\text{H}}_{2}}\text{C}{{\text{O}}_{3}}$的电离常数为${{K}_{\text{al}}}=4.4\times {{10}^{-7}}$、${{K}_{\text{a2}}}=4.4\times {{10}^{-11}}$,${{K}_{\text{sp}}}\left( \text{CaC}{{\text{O}}_{3}} \right)=3.0\times {{10}^{-9}}$、${{K}_{\text{sp}}}\left[ \text{Ca}{{\left( \text{OH} \right)}_{2}} \right]=5.0\times {{10}^{-6}}$。
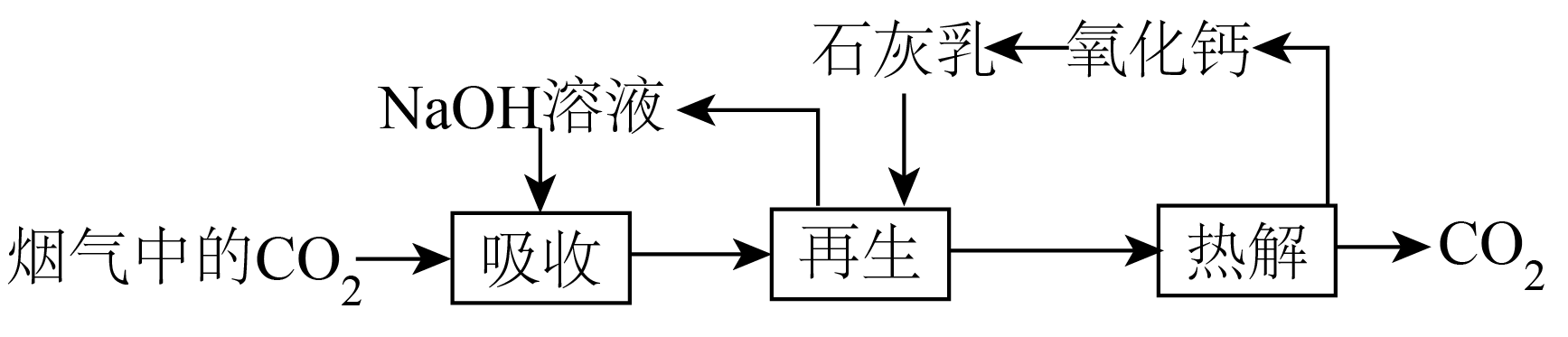
下列说法错误的是$(\quad\ \ \ \ )$
吸收液中$c\\left( {{\\text{H}}_{2}}\\text{C}{{\\text{O}}_{3}} \\right)+c\\left( \\text{HCO}_{3}^{-} \\right)+c\\left( \\text{CO}_{3}^{2-} \\right)=0.1\\,\\text{mol}\\cdot {{\\text{L}}^{-1}}$时,溶液中$c\\left( {{\\text{H}}_{2}}\\text{C}{{\\text{O}}_{3}} \\right)\\gt c\\left( \\text{CO}_{3}^{2-} \\right)$
","吸收液的$\\text{pH}=10$时,$\\dfrac{c\\left( \\text{CO}_{3}^{2-} \\right)}{c\\left( \\text{HCO}_{3}^{-} \\right)}=0.44$
","$\\text{NaOH}$完全转化为$\\text{N}{{\\text{a}}_{2}}\\text{C}{{\\text{O}}_{3}}$时,溶液中$c\\left( \\text{O}{{\\text{H}}^{-}} \\right)=c\\left( {{\\text{H}}^{+}} \\right)+c\\left( \\text{HCO}_{3}^{-} \\right)+2c\\left( {{\\text{H}}_{2}}\\text{C}{{\\text{O}}_{3}} \\right)$
","“再生”后过滤,所得滤液中$\\dfrac{c\\left( \\text{CO}_{3}^{2-} \\right)}{c\\left( \\text{O}{{\\text{H}}^{-}} \\right)}=6.0\\times {{10}^{-4}}$
"]$\rm A$.以$0.1\ \text{mol}\cdot {{\text{L}}^{-1}}$的$\text{NaOH}$溶液吸收捕集烟气中的$\text{C}{{\text{O}}_{2}}$,当吸收液中$c\left( {{\text{H}}_{2}}\text{C}{{\text{O}}_{3}} \right)+c\left( \text{HCO}_{3}^{-} \right)+c\left( \text{CO}_{3}^{2-} \right)=0.1\ \text{mol}\cdot {{\text{L}}^{-1}}$时,此时刚好为$\rm NaHCO_{3}$溶液,$\text{HCO}_{\text{3}}^{-}$的电离常数为${{K}_{\text{a2}}}=4.4\times {{10}^{-11}}$,水解常数为${{K}_{\text{h}2}}=\dfrac{1\times {{10}^{-14}}}{4.4\times {{10}^{-7}}}$,水解常数大于电离常数,故$c\left( {{\text{H}}_{2}}\text{C}{{\text{O}}_{3}} \right)\gt c\left( \text{CO}_{3}^{2-} \right)$,故$\rm A$正确;
$\rm B$.${{K}_{\text{a}2}}=\dfrac{c\left( \text{CO}_{3}^{2-} \right)\text{c(}{{\text{H}}^{+}}\text{)}}{c\left( \text{HCO}_{3}^{-} \right)}=4.4\times {{10}^{-11}}$,$\dfrac{c\left( \text{CO}_{3}^{2-} \right)}{c\left( \text{HCO}_{3}^{-} \right)}=\dfrac{{{K}_{\text{a2}}}}{c\text{(}{{\text{H}}^{+}}\text{)}}=\dfrac{4.4\times {{10}^{-11}}}{1\times {{10}^{-10}}}=0.44$,故$\rm B$正确;
$\rm C$.$\text{NaOH}$完全转化为$\text{N}{{\text{a}}_{2}}\text{C}{{\text{O}}_{3}}$时,根据质子守恒,有等式:$c\left( \text{O}{{\text{H}}^{-}} \right)=c\left( {{\text{H}}^{+}} \right)+c\left( \text{HCO}_{3}^{-} \right)+2c\left( {{\text{H}}_{2}}\text{C}{{\text{O}}_{3}} \right)$,故$\rm C$正确;
$\rm D$.$\dfrac{c\left( \text{CO}_{3}^{2-} \right)}{{{c}^{2}}\left( \text{O}{{\text{H}}^{-}} \right)}=\dfrac{c\left( \text{CO}_{\text{3}}^{\text{2-}} \right)c\text{(C}{{\text{a}}^{\text{2+}}}\text{)}}{{{c}^{\text{2}}}\left( \text{O}{{\text{H}}^{-}} \right)c\text{(C}{{\text{a}}^{\text{2+}}}\text{)}}=\dfrac{{{K}_{\text{sp}}}\text{(CaC}{{\text{O}}_{\text{3}}}\text{)}}{{{K}_{\text{sp}}}[\!\!\text{ Ca(OH}{{\text{)}}_{\text{2}}}]}=\dfrac{3.0\times {{10}^{-9}}}{5.0\times {{10}^{-6}}}=6.0\times {{10}^{-4}}$,故$\rm D$错误;
故选:$\rm D$
高中 | 盐溶液微粒间的三大守恒原理的理解及应用题目答案及解析(完整版)
