高中 | 盐溶液微粒间的三大守恒原理的理解及应用 题目答案及解析
稿件来源:高途
高中 | 盐溶液微粒间的三大守恒原理的理解及应用题目答案及解析如下,仅供参考!
选修四
第三章 水溶液中的离子平衡
第三节 盐类的水解
盐溶液微粒间的三大守恒原理的理解及应用
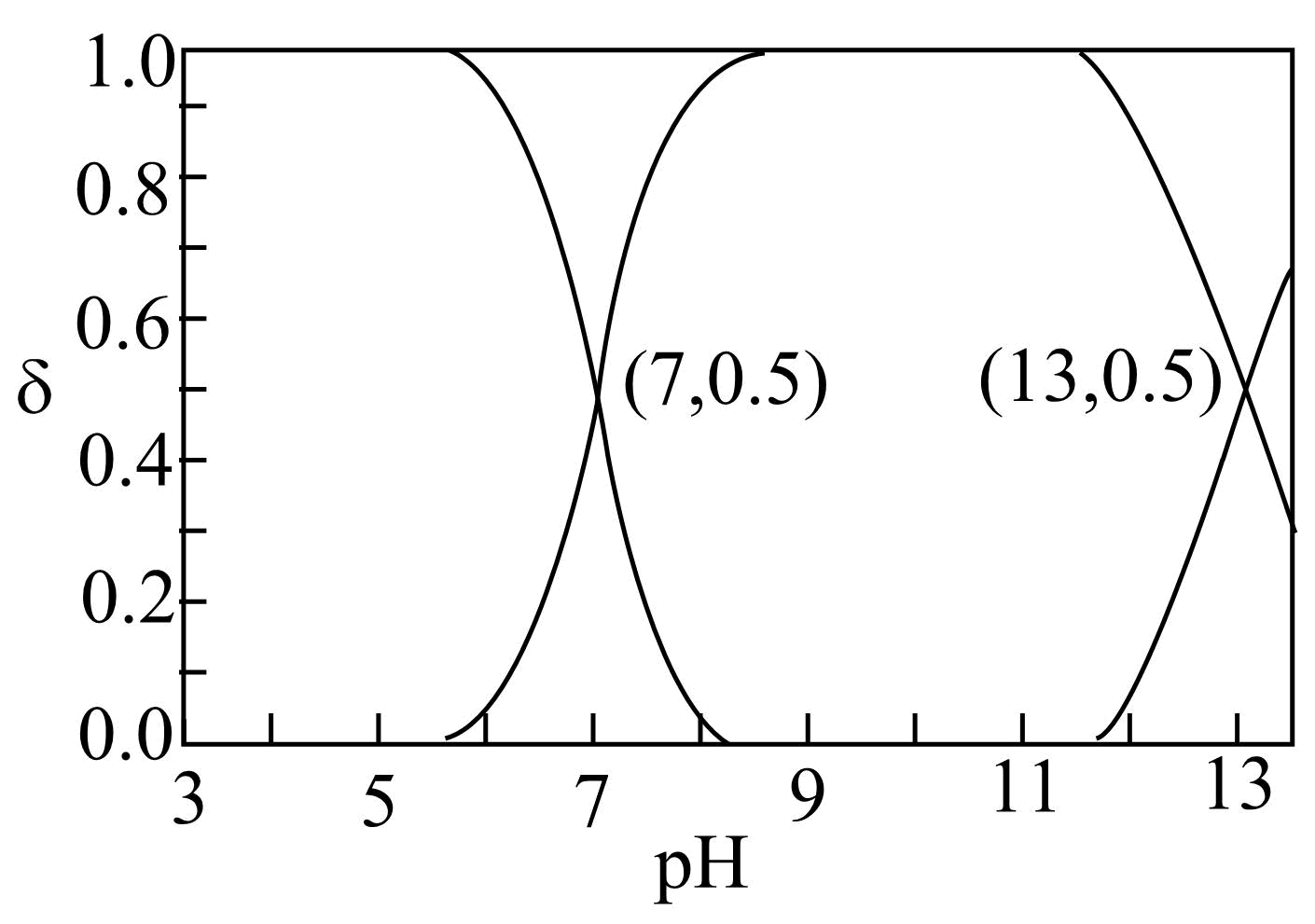
$25\;^\circ \rm C $时,某酸${{\text{H}}_{2}}\text{R}$溶液中存在的各种含$\rm R$元素微粒在总浓度中所占分数$\delta $随溶液$\text{pH}$的变化关系如图所示。下列叙述不正确的是$(\qquad)$
${{ {K}}_{\\text{a2}}}$的数量级为${{10}^{-13}}$
","$\\text{NaHR}$溶液中,${c}\\left( {{\\text{H}}_{2}}\\text{R} \\right)\\gt {c}\\left( {{\\text{R}}^{2-}} \\right)$
","若${c}\\left( {{\\text{H}}_{2}}\\text{R} \\right)+2{c}\\left( {{\\text{R}}^{2-}} \\right)+{c}\\left( \\text{O}{{\\text{H}}^{-}} \\right)={c}\\left( {{\\text{H}}^{+}} \\right)$,则${c}\\left( {{\\text{H}}_{2}}\\text{R} \\right)={c}\\left( \\text{H}{{\\text{R}}^{-}} \\right)$
","$\\text{F}{{\\text{e}}^{2+}}$易与溶液中的${{\\text{R}}^{2-}}$形成沉淀,$\\text{pH}=8$时$\\text{F}{{\\text{e}}^{2+}}$恰好沉淀完全$\\left[ {c}\\left( \\text{F}{{\\text{e}}^{2+}} \\right)=1.0\\times {{10}^{-5}}\\;\\rm \\text{mol}/\\text{L} \\right]$,此时${c}\\left( {{\\text{H}}_{2}}\\text{R} \\right)=6.0\\times {{10}^{-9}}\\;\\rm \\text{mol}/\\text{L}\\rm (\\text{FeR}$的${{ {K}}_{\\text{sp}}}$为$6.0\\times {{10}^{-18}}\\rm )$
"]${{\text{H}}_{2}}\text{R}$的电离方程式为${{\text{H}}_{\text{2}}}\text{R}\rightleftharpoons {{\text{H}}^{+}}\text{+H}{{\text{R}}^{-}}$,$\text{H}{{\text{R}}^{-}}\rightleftharpoons {{\text{H}}^{+}}+{{\text{R}}^{{2-}}}$、,溶液酸性较强时主要发生第一步电离,随着酸性减弱发生第二步电离,由图可知,$\rm pH=7$时, ${c}\left( {{\text{H}}_{\text{2}}}\text{R} \right)=c\left( \text{H}{{\text{R}}^{-}} \right)$,${{ {K}}_{\text{a1}}}=c\left( {{\text{H}}^{+}} \right)\text{=1}{{\text{0}}^{{-7}}}$;$\rm pH=13$时${c}\left( \text{H}{{\text{R}}^{-}} \right)=c\left( {{\text{R}}^{{2-}}} \right)$,${{ {K}}_{\text{a2}}}=\dfrac{c({{\text{H}}^{+}})\cdot c({{\text{R}}^{{2-}}})}{c(\text{H}{{\text{R}}^{-}})}=c\left( {{\text{H}}^{+}} \right)=\text{1}\times \text{1}{{\text{0}}^{{-13}}}$。
$\rm A$. ${{{K}}_{\text{a2}}}=\dfrac{c({{\text{H}}^{+}})\cdot c({{\text{R}}^{{2-}}})}{c(\text{H}{{\text{R}}^{-}})}=c\left( {{\text{H}}^{+}} \right)=\text{1}\times \text{1}{{\text{0}}^{{-13}}}$,$\rm A$正确;
$\rm B$.在$\text{NaHR}$溶液中存在$\text{H}{{\text{R}}^{-}}+{{\text{H}}_{\text{2}}}\text{O}\rightleftharpoons {{\text{H}}_{\text{2}}}\text{R+O}{{\text{H}}^{-}}$,${{ {K}}_{\text{a2}}}=\text{1}\times \text{1}{{\text{0}}^{{-13}}}$,也存在$\text{H}{{\text{R}}^{-}}+{{\text{H}}_{\text{2}}}\text{O}\rightleftharpoons {{\text{H}}_{\text{2}}}\text{R+O}{{\text{H}}^{-}}$,${{ {K}}_{\text{h}}}=\dfrac{c(\text{O}{{\text{H}}^{-}})\cdot c({{\text{H}}_{\text{2}}}\text{R})}{c(\text{H}{{\text{R}}^{-}})}=\dfrac{c\left( {{\text{H}}^{+}} \right)c(\text{O}{{\text{H}}^{-}})\cdot c({{\text{H}}_{\text{2}}}\text{R})}{c(\text{H}{{\text{R}}^{-}})c\left( {{\text{H}}^{+}} \right)}=\dfrac{{{ {K}}_{\text{w}}}}{{{ {K}}_{\rm a1}}}=\dfrac{\text{1}{{\text{0}}^{{-14}}}}{\text{1}{{\text{0}}^{{-7}}}}=\text{1}{{\text{0}}^{{-7}}}$,所以水解大于电离,${c}\left( {{\text{H}}_{2}}\text{R} \right)\gt {c}\left( {{\text{R}}^{2-}} \right)$,$\rm B$正确;
$\rm C$.由图可知当${c}\left( {{\text{H}}_{2}}\text{R} \right)={c}\left( \text{H}{{\text{R}}^{-}} \right)$时溶液显中性${c}\left( {{\text{H}}^{+}} \right)=c\left( \text{O}{{\text{H}}^{-}} \right)$,$\rm C$错误;
$\rm D$.此时${c}\left( {{\text{H}}_{\text{2}}}\text{R} \right)\text{=6}\text{.0}\times \text{1}{{\text{0}}^{{-9}}}\text{ mol/L}$,$\text{pH}=8$,根据${{ {K}}_{\text{a1}}}\cdot {{ {K}}_{\text{a2}}}=\dfrac{{{{c}}^{\text{2}}}\text{(}{{\text{H}}^{+}}\text{)}\times {c(}{{\text{R}}^{{2-}}}\text{)}}{ {c(}{{\text{H}}_{\text{2}}}\text{R)}}\text{=1}{{\text{0}}^{{-7}}}\cdot \text{1}{{\text{0}}^{{-13}}}\text{=1}{{\text{0}}^{{-20}}}$,${c}\left( {{\text{R}}^{{2-}}} \right)=\dfrac{\text{1}{{\text{0}}^{{-20}}}\times \text{6}\text{.0}\times \text{1}{{\text{0}}^{{-9}}}}{{{{c}}^{\text{2}}}\text{(}{{\text{H}}^{+}}\text{)}}=\dfrac{\text{6}\text{.0}\times \text{1}{{\text{0}}^{{-29}}}}{{{{c}}^{\text{2}}}\text{(}{{\text{H}}^{+}}\text{)}}$,${c}\left( \text{F}{{\text{e}}^{\text{2+}}} \right)\cdot {c}\left( {{\text{R}}^{{2-}}} \right)\text{=0}\text{.1}\times \text{1}{{\text{0}}^{{-5}}}\times \dfrac{\text{6}\text{.0}\times \text{1}{{\text{0}}^{{-29}}}}{{{{c}}^{\text{2}}}\text{(}{{\text{H}}^{+}}\text{)}}\text{=6}\text{.0}\times \text{1}{{\text{0}}^{{-18}}}$解得${c}\left( {{\text{H}}^{+}} \right)\text{=1}\times \text{1}{{\text{0}}^{{-8}}}{\text{ mol}}/{\text{L}}$,$\rm D$正确。
故选:$\rm C$
高中 | 盐溶液微粒间的三大守恒原理的理解及应用题目答案及解析(完整版)
