高中 | 盐类水解常数 题目答案及解析
稿件来源:高途
高中 | 盐类水解常数题目答案及解析如下,仅供参考!
选修四
第三章 水溶液中的离子平衡
第三节 盐类的水解
盐类水解常数
常温下,向$\text{N}{{\text{H}}_{4}}\text{F}$溶液中滴加$\rm NaOH$溶液,混合溶液中$\text{lg}X\rm (X$表示$\dfrac{c\left( {{\text{F}}^{-}} \right)}{c(\text{HF})}$或$\dfrac{c\left( \text{NH}_{4}^{+} \right)}{c\left( \text{N}{{\text{H}}_{3}}\cdot {{\text{H}}_{2}}\text{O} \right)}\rm )$随$\rm pH$变化如图,已知${{K}_{\text{a}}}(\text{HF})={{10}^{-4.5}}$,Ⅰ线和Ⅱ线交点横坐标为$\rm 6.6$,下列说法错误的是$(\quad\ \ \ \ )$
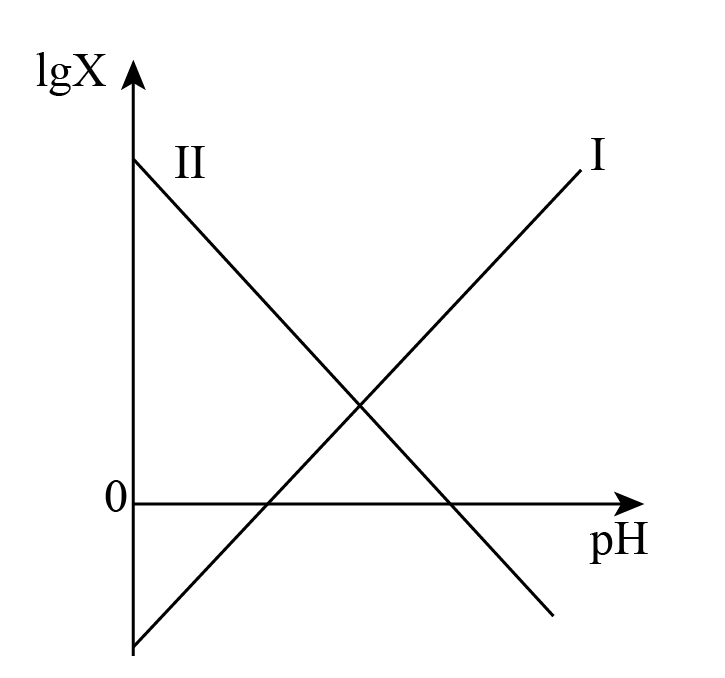
Ⅰ线代表$\\lg \\dfrac{c\\left( {{\\text{F}}^{-}} \\right)}{c(\\text{HF})}$
","Ⅱ线和横轴交点坐标为$\\left( 8.7,0 \\right)$
","$\\text{N}{{\\text{H}}_{4}}\\text{F}$溶液加水稀释过程中$\\rm pH$值逐渐减小
","$\\text{N}{{\\text{H}}_{4}}\\text{F}$溶液中存在:$c\\left( \\text{NH}_{4}^{+} \\right)+c\\left( \\text{O}{{\\text{H}}^{-}} \\right)+2c\\left( \\text{N}{{\\text{H}}_{3}}\\cdot {{\\text{H}}_{2}}\\text{O} \\right)=c\\left( {{\\text{H}}^{+}} \\right)+c\\left( {{\\text{F}}^{-}} \\right)+2c(\\text{HF})$
"]分析:向$\text{N}{{\text{H}}_{4}}\text{F}$溶液中滴加$\rm NaOH$溶液,随着$\rm PH$增大,$c\left( {{\text{F}}^{-}} \right)$增大$c\left( \text{H}{{\text{F}}^{{}}} \right)$减小,$\text{lg}\dfrac{c\left( {{\text{F}}^{-}} \right)}{c(\text{HF})}$增大, $c\left( \text{NH}_{4}^{+} \right)$减小$c\left( \text{N}{{\text{H}}_{3}}\cdot {{\text{H}}_{2}}\text{O} \right)$增大,$\text{lg}\dfrac{c\left( \text{NH}_{4}^{+} \right)}{c\left( \text{N}{{\text{H}}_{3}}\cdot {{\text{H}}_{2}}\text{O} \right)}$减小。
$\rm A$.Ⅰ线代表$\lg \dfrac{c\left( {{\text{F}}^{-}} \right)}{c(\text{HF})}$,$\rm A$正确;
$\rm B$.Ⅰ线和Ⅱ线交点横坐标为$\rm 6.6$可得$\dfrac{c\left( {{\text{F}}^{-}} \right)}{c\text{(HF)}}=\dfrac{{{\text{K}}_{\text{a}}}}{c\text{(}{{\text{H}}^{+}}\text{)}}=\dfrac{{{K}_{\text{b}}}}{c\text{(O}{{\text{H}}^{-}}\text{)}}=\dfrac{c\left( \text{NH}_{4}^{+} \right)}{c\left( \text{N}{{\text{H}}_{3}}\cdot {{\text{H}}_{2}}\text{O} \right)}\Rightarrow \dfrac{\text{1}{{\text{0}}^{\text{-4}\text{.5}}}}{\text{1}{{\text{0}}^{\text{-6}\text{.6}}}}=\dfrac{{{K}_{\text{b}}}}{\text{1}{{\text{0}}^{\text{6}\text{.6}}}^{-14}}\Rightarrow {{K}_{\text{b}}}={{10}^{-5.3}}$,横轴交点说明$\dfrac{c\left( \text{NH}_{4}^{+} \right)}{c\left( \text{N}{{\text{H}}_{3}}\cdot {{\text{H}}_{2}}\text{O} \right)}\rm =1$则$\dfrac{{{K}_{\text{b}}}}{c\text{(O}{{\text{H}}^{-}}\text{)}}=\dfrac{c\left( \text{NH}_{4}^{+} \right)}{c\left( \text{N}{{\text{H}}_{3}}\cdot {{\text{H}}_{2}}\text{O} \right)}\Rightarrow c\text{(O}{{\text{H}}^{-}}\text{)}={{10}^{-5.3}}$,$\text{pH}=8.7$,$\rm B$正确;
$\rm C$.${{K}_{\text{a}}}(\text{HF})={{10}^{-4.5}}$,$\rm F^{-}$的水解平衡常数${{K}_{\text{h}}}=\dfrac{{{K}_{\text{w}}}}{{{K}_{\text{a}}}}=\dfrac{{{10}^{-14}}}{{{10}^{-4.5}}}={{10}^{-9.5}}$,$\text{NH}_{\text{4}}^{+}$的水解平衡常数${{K}_{\text{h}}}=\dfrac{{{K}_{\text{w}}}}{{{K}_{\text{a}}}}=\dfrac{{{10}^{-14}}}{{{10}^{-5.3}}}={{10}^{-8.7}}$,$\text{N}{{\text{H}}_{4}}\text{F}$溶液显酸性,加水稀释过程中水解程度增大,但氢离子浓度减小,$\rm pH$值逐渐增大,$\rm C$错误;
$\rm D$.$\text{N}{{\text{H}}_{4}}\text{F}$溶液中存在:电荷平衡$c\left( \text{NH}_{4}^{+} \right)+c\left( {{\text{H}}^{+}} \right)=c\left( {{\text{F}}^{-}} \right)+c\left( \text{O}{{\text{H}}^{-}} \right)$,物料守恒
$c\left( \text{NH}_{4}^{+} \right)+c\left( \text{N}{{\text{H}}_{3}}\cdot {{\text{H}}_{2}}\text{O} \right)=c\left( {{\text{F}}^{-}} \right)+c(\text{HF})$,两式相减得$c\left( \text{O}{{\text{H}}^{-}} \right)+c\left( \text{N}{{\text{H}}_{3}}\cdot {{\text{H}}_{2}}\text{O} \right)=c\left( {{\text{H}}^{+}} \right)+c(\text{HF})$,与$c\left( \text{NH}_{4}^{+} \right)+c\left( \text{N}{{\text{H}}_{3}}\cdot {{\text{H}}_{2}}\text{O} \right)=c\left( {{\text{F}}^{-}} \right)+c(\text{HF})$相加得$c\left( \text{NH}_{4}^{+} \right)+c\left( \text{O}{{\text{H}}^{-}} \right)+2c\left( \text{N}{{\text{H}}_{3}}\cdot {{\text{H}}_{2}}\text{O} \right)=c\left( {{\text{H}}^{+}} \right)+c\left( {{\text{F}}^{-}} \right)+2c(\text{HF})$,$\rm D$正确;
故选:$\rm C$
高中 | 盐类水解常数题目答案及解析(完整版)
