高中 | 盐溶液微粒间的三大守恒原理的理解及应用 题目答案及解析
稿件来源:高途
高中 | 盐溶液微粒间的三大守恒原理的理解及应用题目答案及解析如下,仅供参考!
选修四
第三章 水溶液中的离子平衡
第三节 盐类的水解
盐溶液微粒间的三大守恒原理的理解及应用
室温下,用$\text{NaOH}$溶液滴定$\rm X(\text{Na}{{\text{H}}_{2}}\text{P}{{\text{O}}_{4}}$或$\text{N}{{\text{H}}_{4}}\text{Cl}\rm )$的稀溶液,溶液$\rm pH$与$\dfrac{ {n}\left( \text{NaOH} \right)}{ {n}\left( \text{X} \right)}$的关系如图所示,已知:$p K=-\lg K$,室温下,下列说法错误的是$(\qquad)$
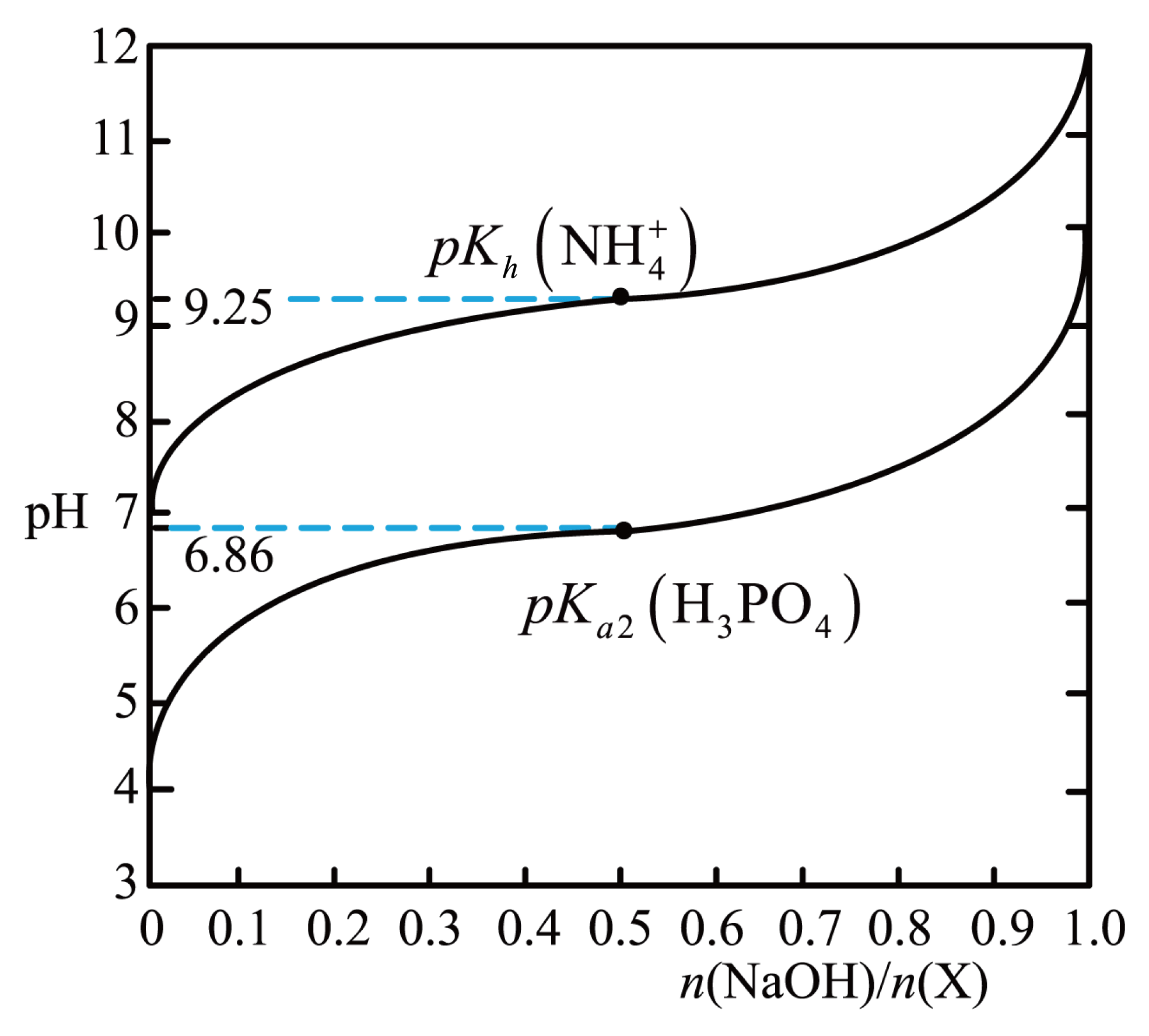
$0.1\\;\\rm \\text{mol}\\cdot {{\\text{L}}^{-1}}\\text{ Na}{{\\text{H}}_{2}}\\text{P}{{\\text{O}}_{4}}$溶液$\\text{pH}\\lt 7$
","$0.1\\;\\rm \\text{mol}\\cdot {{\\text{L}}^{-1}}\\text{ N}{{\\text{H}}_{4}}\\text{Cl}$溶液中滴加氨水至溶液呈中性:$c\\left( \\text{C}{{\\text{l}}^{-}} \\right)={c}\\left( \\text{NH}_{4}^{+} \\right)\\lt 100{c}\\left( \\text{N}{{\\text{H}}_{3}}\\cdot {{\\text{H}}_{2}}\\text{O} \\right)$
","$0.1\\;\\rm \\text{mol}\\cdot {{\\text{L}}^{-1}}\\text{ Na}{{\\text{H}}_{2}}\\text{P}{{\\text{O}}_{4}}$溶液中滴加$\\text{NaOH}$至溶液呈中性:${c}\\left( \\text{N}{{\\text{a}}^{+}} \\right)\\lt 3{c}\\left( \\text{HPO}_{4}^{2-} \\right)+3{c}\\left( \\text{PO}_{4}^{3-} \\right)$
","$0.1\\;\\rm \\text{mol}\\cdot {{\\text{L}}^{-1}}\\text{ Na}{{\\text{H}}_{2}}\\text{P}{{\\text{O}}_{4}}$溶液中滴加${{\\text{K}}_{\\text{3}}}\\text{P}{{\\text{O}}_{\\text{4}}}$至溶液呈中性:$c\\left( {{\\text{K}}^{+}} \\right)\\gt c\\left( \\text{N}{{\\text{a}}^{+}} \\right)$
"]$\rm A$.图像可知,当起点时,即还没有滴加$\rm NaOH$溶液时,为$\text{Na}{{\text{H}}_{2}}\text{P}{{\text{O}}_{4}}$溶液,溶液$\text{pH}\lt 7$,选项$\rm A$正确;
$\rm B$.$0.1\;\rm \text{mol}\cdot {{\text{L}}^{-1}}\text{ N}{{\text{H}}_{4}}\text{Cl}$溶液中,由电荷守恒得$c\left( \text{NH}_{4}^{+} \right)+c\left( {{\text{H}}^{+}} \right)=c\left( \text{C}{{\text{l}}^{-}} \right)+c\left( \text{O}{{\text{H}}^{-}} \right)$,溶液呈中性,即有$c\left( {{\text{H}}^{+}} \right)=c\left( \text{O}{{\text{H}}^{-}} \right)$,故$c\left( \text{NH}_{4}^{+} \right)=c\left( \text{C}{{\text{l}}^{-}} \right)$,$\text{NH}_{4}^{+}$水解显酸性,$\text{N}{{\text{H}}_{\text{3}}}\cdot {{\text{H}}_{\text{2}}}\text{O}$电离显碱性,由图像可知$\dfrac{ {n}\left( \text{NaOH} \right)}{ {n}\left( \text{NH}_{4}^{+} \right)}=0.5$时,即$\text{NH}_{4}^{+}$与$\text{N}{{\text{H}}_{\text{3}}}\cdot {{\text{H}}_{\text{2}}}\text{O}$为$\rm 1: 1$时,$\rm pH=9.25$,$c\left( \text{O}{{\text{H}}^{-}} \right)=\text{1}{{\text{0}}^{{-4}\text{.75}}}\text{ mol/L}$,$ K_{\rm b}=\dfrac{c(\text{NH}_{\text{4}}^{+})\cdot c(\text{O}{{\text{H}}^{{-}}})}{c(\text{N}{{\text{H}}_{\text{3}}}\cdot {{\text{H}}_{\text{2}}}\text{O})}=c(\text{O}{{\text{H}}^{{-}}})=\rm 10^{-4.75}\;\rm mol/L$,当溶液呈中性时,即$\rm pH=7$时,$ K_{\rm b}=\dfrac{c(\text{NH}_{\text{4}}^{+})\times {{10}^{-7}}}{c(\text{N}{{\text{H}}_{\text{3}}}\cdot {{\text{H}}_{\text{2}}}\text{O})}=\rm 10^{-4.75}\;\rm mol/L$,$\dfrac{c(\text{NH}_{\text{4}}^{+})}{c(\text{N}{{\text{H}}_{\text{3}}}\cdot {{\text{H}}_{\text{2}}}\text{O})}=\rm 10^{2.25}$,${c}\left( \text{NH}_{4}^{+} \right)={{10}^{2.25}}{c}\left( \text{N}{{\text{H}}_{3}}\cdot {{\text{H}}_{2}}\text{O} \right)\gt 100{c}\left( \text{N}{{\text{H}}_{3}}\cdot {{\text{H}}_{2}}\text{O} \right)$,选项$\rm B$错误;
$\rm C$.$0.1\;\rm \text{mol}\cdot {{\text{L}}^{-1}}\text{ Na}{{\text{H}}_{2}}\text{P}{{\text{O}}_{4}}$溶液中,由电荷守恒得:${c}\left( \text{N}{{\text{a}}^{+}} \right)+c\left( {{\text{H}}^{+}} \right)={c}\left( {{\text{H}}_{\text{2}}}\text{PO}_{4}^{-} \right)+2{c}\left( \text{HPO}_{4}^{2-} \right)+3{c}\left( \text{PO}_{4}^{3-} \right)+c\left( \text{O}{{\text{H}}^{-}} \right)$,溶液呈中性,即有$ {c}\left(\mathrm{H}^{+}\right)= {c}\left(\mathrm{OH}^{-}\right)$,故${c}\left( \text{N}{{\text{a}}^{+}} \right)={c}\left( {{\text{H}}_{\text{2}}}\text{PO}_{4}^{-} \right)+2{c}\left( \text{HPO}_{4}^{2-} \right)+3{c}\left( \text{PO}_{4}^{3-} \right)$,图像可知,当时$\dfrac{ {n}\left( \text{NaOH} \right)}{ {n}\left( {{\text{H}}_{\text{2}}}\text{PO}_{4}^{-} \right)}=0.5$,即${{\text{H}}_{\text{2}}}\text{PO}_{4}^{-}$与$\text{HPO}_{4}^{2-}$为$\rm 1:1$时,溶液呈酸性$\rm (pH=6.86)$,若溶液呈中性,$\text{HPO}_{4}^{2-}$的量需要稍大,即有${c}\left( \text{N}{{\text{a}}^{+}} \right)\lt 3{c}\left( \text{HPO}_{4}^{2-} \right)+3{c}\left( \text{PO}_{4}^{3-} \right)$,选项$\rm C$正确;
$\rm D$.当$\text{Na}{{\text{H}}_{2}}\text{P}{{\text{O}}_{4}}$与${{\text{K}}_{\text{3}}}\text{P}{{\text{O}}_{\text{4}}}$为$\rm 3:1$时,溶液中$c\left( {{\text{K}}^{+}} \right)=c\left( \text{N}{{\text{a}}^{+}} \right)$,由于${{\text{H}}_{\text{2}}}\text{PO}_{4}^{-}+\text{PO}_{4}^{3-}\rightleftharpoons 2\text{HPO}_{4}^{2-}$,此时溶液中${{\text{H}}_{\text{2}}}\text{PO}_{4}^{-}$与$\text{HPO}_{4}^{2-}$为$\rm 1:1$,由图像可知,此时溶液呈酸性,$\rm pH=6.86$,若溶液呈中性,$\rm{{ {K}}_{\text{3}}}\text{P}{{\text{O}}_{\text{4}}}$的量需要稍微大一点,故$c\left( \rm{{ {K}}^{+}} \right)\gt c\left( \text{N}{{\text{a}}^{+}} \right)$,选项$\rm D$正确。
故选:$\rm B$
高中 | 盐溶液微粒间的三大守恒原理的理解及应用题目答案及解析(完整版)
