| 化学平衡常数 题目答案及解析
稿件来源:高途
| 化学平衡常数题目答案及解析如下,仅供参考!
选修四
第二章 化学反应速率和化学平衡
第三节 化学平衡
化学平衡常数
$\rm 25\;\rm ^\circ\rm C$时,$0.1\,\text{mol}/\text{L}\,{{\text{K}}_{2}}\text{C}{{\text{r}}_{2}}{{\text{O}}_{7}}$溶液中存在以下平衡;
①$\text{C}{{\text{r}}_{2}}\text{O}_{7}^{2-}\left( \text{aq} \right)+{{\text{H}}_{2}}\text{O}\left( \text{l} \right)\rightleftharpoons 2\text{CrO}_{4}^{2-}\left( \text{aq} \right)+2{{\text{H}}^{+}}\left( \text{aq} \right)\quad {{K}_{1}}=3.27\times {{10}^{-15}}$
②$\text{C}{{\text{r}}_{2}}\text{O}_{7}^{2-}\left( \text{aq} \right)+{{\text{H}}_{2}}\text{O}\left( \text{l} \right)\rightleftharpoons 2\text{HCrO}_{4}^{-}\left( \text{aq} \right)\quad {{K}_{2}}$
③$\text{HCrO}_{4}^{-}\left( \text{aq} \right)\rightleftharpoons \text{CrO}_{4}^{2-}\left( \text{aq} \right)+{{\text{H}}^{+}}\left( \text{aq} \right)\quad {{K}_{3}}=3.3\times {{10}^{-7}}$
其中$\text{lg}\,\dfrac{c\left( \text{CrO}_{4}^{2-} \right)}{c\left( \text{C}{{\text{r}}_{2}}\text{O}_{7}^{2-} \right)}$随$\rm pH$的变化关系如图所示。下列说法错误的是$(\quad\ \ \ \ )$
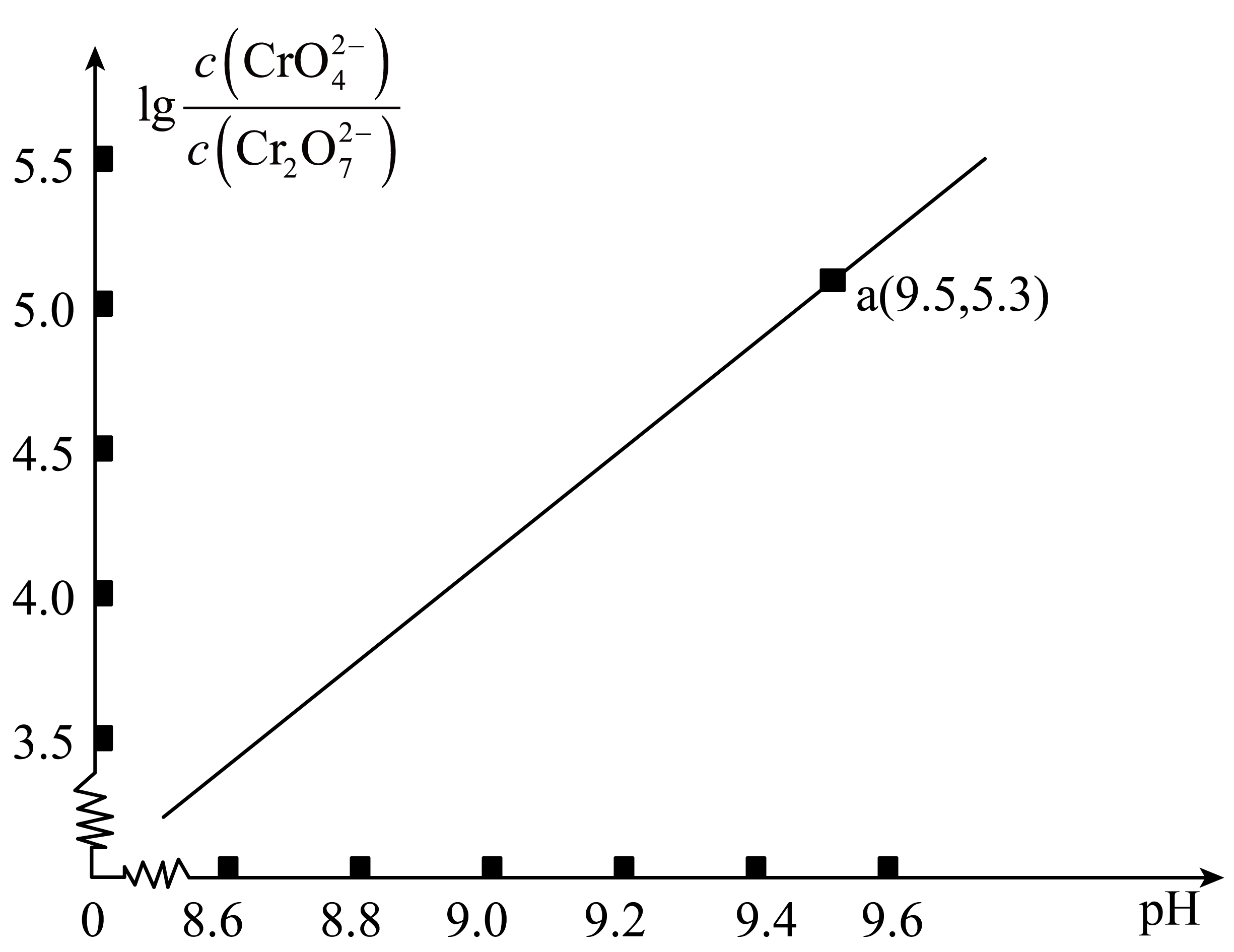
$\\rm pH$增大,$\\dfrac{c\\left( {{\\text{H}}^{+}} \\right)\\cdot {{c}^{2}}\\left( \\text{CrO}_{4}^{2-} \\right)}{c\\left( \\text{C}{{\\text{r}}_{2}}\\text{O}_{7}^{2-} \\right)}$的值减小
","反应③的化学平衡常数${{K}_{3}}={{\\left( \\dfrac{{{K}_{1}}}{{{K}_{2}}} \\right)}^{\\dfrac{1}{2}}}$
","$\\text{pH}=9.5$时,$\\text{HCrO}_{4}^{-}$的浓度约为$1.0\\times {{10}^{-3.8}}\\,\\text{mol}/\\text{L}$
","$\\rm a$点溶液中离子浓度关系;$c\\left( {{\\text{K}}^{+}} \\right)\\gt c\\left( \\text{CrO}_{4}^{2-} \\right)\\gt c\\left( \\text{C}{{\\text{r}}_{2}}\\text{O}_{7}^{2-} \\right)\\gt c\\left( {{\\text{H}}^{+}} \\right)$
"]$\rm A$.${{K}_{1}}=\dfrac{{{c}^{2}}\left( {{\text{H}}^{+}} \right)\cdot {{c}^{2}}\left( \text{CrO}_{4}^{2-} \right)}{c\left( \text{C}{{\text{r}}_{2}}\text{O}_{7}^{2-} \right)}$,$\dfrac{c\left( {{\text{H}}^{+}} \right)\cdot {{c}^{2}}\left( \text{CrO}_{4}^{2-} \right)}{c\left( \text{C}{{\text{r}}_{2}}\text{O}_{7}^{2-} \right)}=\dfrac{{{K}_{1}}}{c\left( {{\text{H}}^{+}} \right)}$,可知$\rm pH$增大$c\left(\mathrm{H}^{+}\right)$减小,平衡常数不变,$\dfrac{c\left( {{\text{H}}^{+}} \right)\cdot {{c}^{2}}\left( \text{CrO}_{4}^{2-} \right)}{c\left( \text{C}{{\text{r}}_{2}}\text{O}_{7}^{2-} \right)}$的值增大,$\rm A$错误;
$\rm B$.反应③$\rm =\dfrac{1}{2}\rm ($①$\rm -$②$\rm )$,则反应③的化学平衡常数${{K}_{3}}={{\left( \dfrac{{{K}_{1}}}{{{K}_{2}}} \right)}^{\dfrac{1}{2}}}$,$\rm B$正确;
$\rm C$.$\text{pH}=9.5$时,$c\left( {{\text{H}}^{+}} \right)={{10}^{-9.5}}\,\text{mol}/\text{L}$,$\dfrac{c\left( \text{CrO}_{4}^{2-} \right)}{c\left( \text{C}{{\text{r}}_{2}}\text{O}_{7}^{2-} \right)}={{10}^{5.3}}$,${{K}_{1}}=\dfrac{{{c}^{2}}\left( {{\text{H}}^{+}} \right)\cdot {{c}^{2}}\left( \text{CrO}_{4}^{2-} \right)}{c\left( \text{C}{{\text{r}}_{2}}\text{O}_{7}^{2-} \right)}=3.27\times {{10}^{-15}}$,求得$c\left( \text{CrO}_{4}^{2-} \right)=3.27\times {{10}^{-1.3}}\,\text{mol}/\text{L}$,${{K}_{3}}=\dfrac{c\left( {{\text{H}}^{+}} \right)\cdot c\left( \text{CrO}_{4}^{2-} \right)}{c\left( \text{HCrO}_{4}^{-} \right)}=\dfrac{{{10}^{-9.5}}\times 3.27\times {{10}^{-1.3}}}{c\left( \text{HCrO}_{4}^{-} \right)}=3.3\times {{10}^{-7}}$,$\text{HCrO}_{4}^{-}$的浓度约为$1.0\times {{10}^{-3.8}}\;\rm \text{mol}/\text{L}$,$\rm C$正确;
$\rm D$.$0.1\,\text{mol}/\text{L}\,{{\text{K}}_{2}}\text{C}{{\text{r}}_{2}}{{\text{O}}_{7}}$溶液中,$c\left( {{\text{K}}^{+}} \right)$最大,$\rm a$点溶液中$\dfrac{c\left( \text{CrO}_{4}^{2-} \right)}{c\left( \text{C}{{\text{r}}_{2}}\text{O}_{7}^{2-} \right)}={{10}^{5.3}}$,$c\left( \text{CrO}_{4}^{2-} \right)\gt c\left( \text{C}{{\text{r}}_{2}}\text{O}_{7}^{2-} \right)$,根据$\rm C$项数据,$c\left( \text{CrO}_{4}^{2-} \right)=3.27\times {{10}^{-1.3}}\;\rm \text{mol}/\text{L}$,$c\left( \text{C}{{\text{r}}_{2}}\text{O}_{7}^{2-} \right)=3.27\times {{10}^{-6.3}}\;\rm \text{mol}/\text{L}$,$c\left( {{\text{H}}^{+}} \right)={{10}^{-9.5}}\;\rm \text{mol}/\text{L}$,可知$\rm a$点溶液中离子浓度关系;$c\left( {{\text{K}}^{+}} \right)\gt c\left( \text{CrO}_{4}^{2-} \right)\gt c\left( \text{C}{{\text{r}}_{2}}\text{O}_{7}^{2-} \right)\gt c\left( {{\text{H}}^{+}} \right)$,$\rm D$正确;
故选:$\rm A$
| 化学平衡常数题目答案及解析(完整版)
